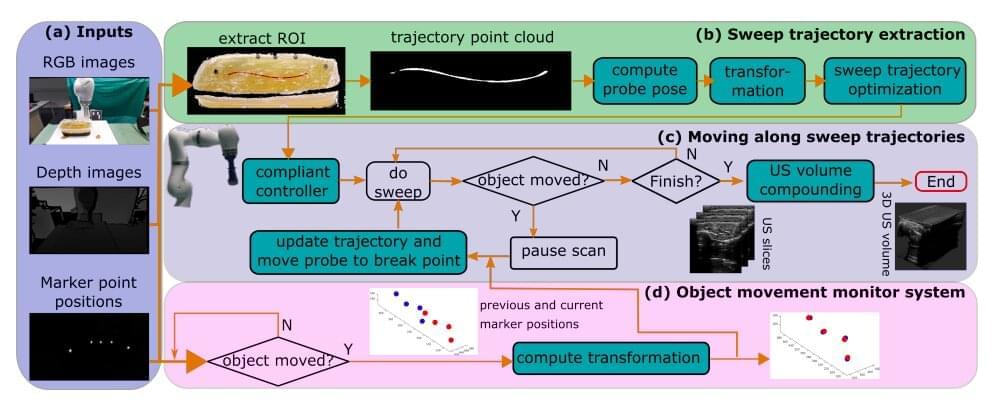
Proteins can communicate through DNA, conducting a long-distance dialogue that serves as a kind of genetic “switch,” according to Weizmann Institute of Science researchers. They found that the binding of proteins to one site of a DNA molecule can physically affect another binding site at a distant location, and that this “peer effect” activates certain genes. This effect had previously been observed in artificial systems, but the Weizmann study is the first to show it takes place in the DNA of living organisms.
A team headed by Dr. Hagen Hofmann of the Chemical and Structural Biology Department made this discovery while studying a peculiar phenomenon in the soil bacteria Bacillus subtilis. A small minority of these bacteria demonstrate a unique skill: an ability to enrich their genomes by taking up bacterial gene segments scattered in the soil around them. This ability depends on a protein called ComK, a transcription factor, which binds to the DNA to activate the genes that make the scavenging possible. However, it was unknown how exactly this activation works.