Widespread pain, a subset of chronic pain associated with musculoskeletal disorders, is linked to an increased risk of all types of dementias, including Alzheimer’s disease, and a greater risk of stroke.


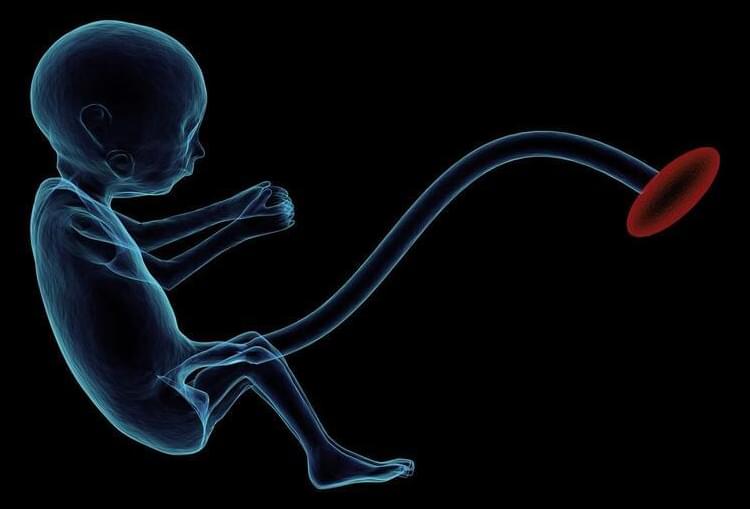
In the study, researchers in the laboratory of Anna Penn, MD, Ph.D., now at Columbia University Vagelos College of Physicians and Surgeons and previously at Children’s National Hospital in Washington, D.C., found that reducing amounts of a single hormone, called allopregnanolone (ALLO), in the placenta caused brain and behavior changes in male offspring that resemble changes seen in some people with autism spectrum disorder. The study also found that both brain structure and behavioral changes in the mice could be prevented with a single injection of ALLO in late pregnancy.
Preterm birth has been shown to increase the risk of autism spectrum disorders and other developmental problems, particularly in males. The more premature a baby is, the greater the risk of either motor or cognitive deficits. What does the preterm baby lose that is so critical to long-term outcomes?
A new study, in mice, suggests that one factor may be the loss of a placental hormone that the developing brain would normally see in the second half of pregnancy.
The study is the first to provide direct evidence that loss of a placental hormone alters long-term brain development.
For the 40-million amputees in need of prosthetic limbs, this personalized and affordable process for fitting prosthetics means hope for a better future.
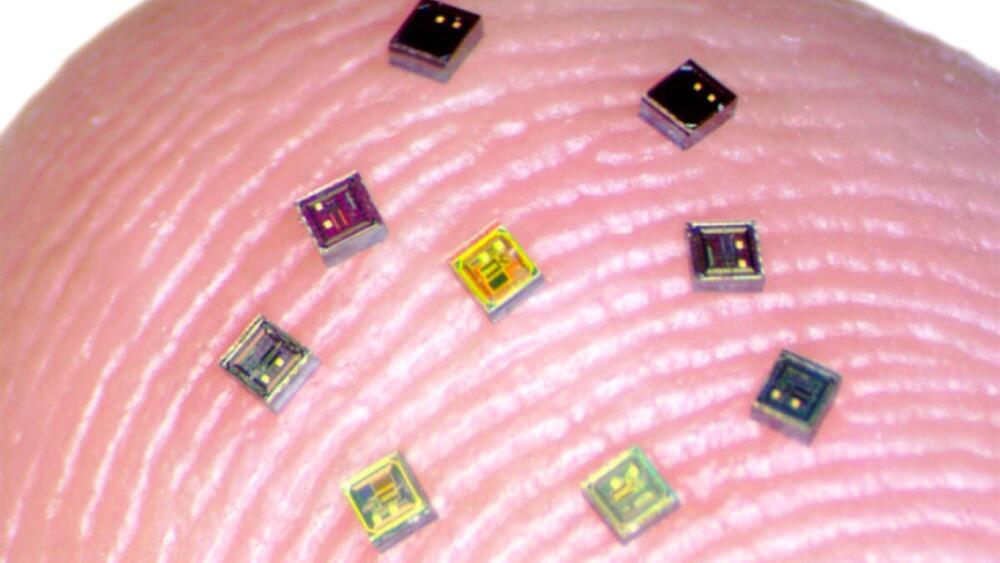
A new kind of brain-computer interface (BCI) that uses neural implants the size of a grain of sand to record brain activity has been proven effective in rats — and one day, thousands of the “neurograins” could help you control machines with your mind.
Mind readers: BCIs are devices (usually electrodes implanted in the skull) that translate electrical signals from brain cells into commands for machines. They can allow paralyzed people to “speak” again, control robots, type with their minds, and even regain control of their own limbs.
Most of today’s interfaces can listen to just a few hundred neurons — but there are approximately 86 billion neurons in the brain. If we could monitor more neurons, in more places in the brain, it could radically upgrade what’s possible with mind-controlled tech.
Artificial gravity for spaceflight is a concept older than spaceflight itself, but we’ve only ever seen one small scale test ever flown in space. However decades of research have been performed to show that the human body can adapt to the conditions required for rotating artificial gravity. This shows that it’s an engineering problem that likely solvable for interested parties who want to spend the time, effort and money creating the classic rotating space stations from Science Fiction.
Here’s a couple of papers which were heavily referenced in researching this.
https://ntrs.nasa.gov/api/citations/19720019454/downloads/19720019454.pdf.
https://ntrs.nasa.gov/api/citations/19730003384/downloads/19730003384.pdf.
The Voyager space station video is from the Gateway Foundation.
https://www.youtube.com/channel/UCfq9IoUJBIKORP6Q0Zp4dIg.
Intro and End segments by Concodroid and Eclipso.
The field of neuroprosthetics was around in its earliest stage in the 1950s, but it’s only just starting to show its true potential, with devices that allow amputees to feel and manipulate their surroundings.
A group of researchers from MIT and Shanghai Jiao Tong University, recently collaborated with the goal of making neuroprosthetic hands, which allow users to feel in a more accessible way. The result is an inflatable robotic hand that costs only $500 to build, making it much cheaper than comparable devices, a post from MIT reveals.
The researchers behind the new prosthetic say their device bears an uncanny resemblance to the inflatable robot in the animated film Big Hero 6. The prosthetic uses a pneumatic system to inflate and bend the fingers of the device, allowing its user to grasp objects, pour a drink, shake hands, and even pet a cat if they so wish. It allows all of this via a software program — detailed in the team’s paper in the journal Nature Biomedical Engineering — that “decodes” EMG signals the brain is sending to an injured or missing limb.

“So many genes are involved in DNA maintenance, FOXO3 for example, which is very interesting, but it cannot be a therapeutic target because it will trigger a lot of other things,” he explains. “SIRT6 is coding for only one protein and, because it’s a small protein, the cargo size is not too big and it can be easily delivered into cells, so it’s possible to use it as a gene therapy target.”
Some of the other factors that play in Genflow’s favour, says Leire, are that the world has reached a better understanding of the biology of aging, but also that gene therapy has also progressed well over the years.
It seems everyday more and more information is being uncovered about trees and the many mysteries within them. We know that they are alive, but it seems they are even more alive than we may have thought. Trees are interconnected underground, we also now know that trees can communicate with one another, but recently scientists have discovered that trees actually have a sort of heartbeat, it is just so slow that they’ve never noticed before.
Up until recently scientists had thought that water moved through trees by the process of osmosis, in a sort of continuous matter, but now they’ve discovered that the trunks and branches of the trees are actually contracting and expanding and essentially pumping water up from the roots to the leaves, kind of like how our heart pumps blood throughout our bodies.
— Our Journalism Is Moving — Our investigative journalism and reporting is moving to our new brand called The Pulse. Click here to stay informed.
But one idea that hasn’t gotten enough attention from the AI community is how the brain creates itself, argues Peter Robin Hiesinger, Professor of Neurobiology at the Free University of Berlin (Freie Universität Berlin).
In his book The Self-Assembling Brain, Hiesinger suggests that instead of looking at the brain from an endpoint perspective, we should study how information encoded in the genome is transformed to become the brain as we grow. This line of study might help discover new ideas and directions of research for the AI community.
The Self-Assembling Brain is organized as a series of seminar presentations interspersed with discussions between a robotics engineer, a neuroscientist, a geneticist, and an AI researcher. The thought-provoking conversations help to understand the views and the holes of each field on topics related to the mind, the brain, intelligence, and AI.