University of Oxford-led research finds low-dose rapamycin functions as a genomic protector in aging human immune cells, lowering DNA damage.
The mechanistic target of rapamycin (mTOR) is a central signaling pathway that regulates and coordinates cell growth, metabolism, and survival in response to environmental cues. It helps cells integrate signals from growth factors, nutrients, and stress to control whether they are in an anabolic (building up) or catabolic (breaking down) state.
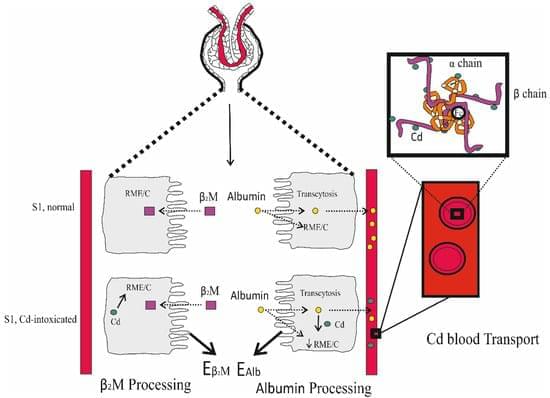
Aging immune systems accumulate DNA damage linked to immunosenescence. Rapamycin is a drug that inhibits the mTOR pathway. Originally developed for organ transplantation to prevent immune rejection, previous research has found that, at non-immunosuppressive doses, rapamycin can mitigate cellular senescence.