Hackers are targeting U.S. hospitals just as COVID-19 cases surge again.


International Health Management, Across 17 Countries, 60 Clinics, and 350 Staff — Dr. James Allen, Health Systems Thinkers, LLC.
Dr. James Allen is a primary care internal medicine specialist who developed a fascinating career in international health management and leadership.
Dr. Allen served in the U.S. public health service before moving to Indonesian Borneo in 1994. For the next 22 years he worked in community and occupational health across Asia, managing health teams in 14 countries. As Chevron’s Asia Pacific medical director, he led projects for TB control in Myanmar, primary care in the Philippines, Indonesia, Vietnam, and Bangladesh; and emergency medicine in Azerbaijan and rural China.
After moving to California headquarters in late 2015, Dr. Allen created a global strategy on corporate responsibility for health, establishing a data-based approach in alignment with the Institute for Health Metrics and Evaluation of Seattle. As Chevron’s senior consulting health scientist, he advised social investment teams in Australasia, Central Europe, Latin America, North America, and West Africa. Dr. Allen completed his career at Chevron in 2021 by leading the implementation of Covid-19 management practices for a consortium of oil and gas companies in Angola.
In 2012, Dr. Allen became an adjunct faculty member for the Levinson Institute’s Strategic Leadership for Healthcare Executives, previously affiliated with Harvard Medical School, and now with Pariveda Solutions and Rice University. His education includes a BA from Antioch College, MS from Rensselaer Polytechnic Institute, and his medical degree from Kirksville, MO. He is certified in internal medicine by the American Board of Internal Medicine, and has completed graduate studies in public health, occupational medicine, tropical medicine, toxicology, and healthcare finance and systems management from various institutions – Cornell, the Medical College of Wisconsin, NY Medical College and Singapore Management Institute.
A couple people from TRIM are in TRIM-X to see how it works a second time.
In this video Dr. Fahy discusses what we can do to make the most of our thymus without the growth hormone treatment, what the timing makes sense for rejuvenation of the thymus and whether the thymus is tied to the other hallmarks of aging.
Dr. Greg Fahy is a world renowned cryobiologist and is also the chief science officer, and co-founder, of Intervene Immune, a company which pioneers treatments for thymus regeneration and age-related immune system decline. Dr. Fahy Designed and led the pilot TRIIM trial which first time showing both thymus rejuvenation and reversal of human epigenetic age. He is now running the follow up phase II trial TRIIM-X with the aim of confirming and extending the results.
************************************************************
Health claims Disclosure: Information provided on this video is not a substitute for direct, individual medical treatment or advice. Please consult with your doctor first. Products or services mentioned in this video are not a recommendation.
Audio Copyright Disclaimer:
Stanford is looking to democratize research on artificial intelligence and medicine by releasing the world’s largest free repository of AI-ready annotated medical imaging datasets. This will allow people from all over the world to access specific data that they need for their respective projects, which could lead to potentially life-saving breakthroughs in these fields.
The use of artificial intelligence in medicine is becoming increasingly pervasive. From analyzing tumors to detecting a person’s pumping heart, AI looks like it will have an important role for the near future.
The AI-powered devices, which can rival the accuracy of human doctors in diagnosing diseases and illnesses, have been making strides as well. These systems not only spot a likely tumor or bone fracture but also predict the course of an illness with some reliability for recommendations on what to do next. However, these systems require expensive datasets that are created by humans who annotate images meticulously before handing them over to compute power, so they’re rather costly either way you look at it given their price tags–millions even if your data is purchased from others or millions more if one has created their own dataset painstakingly through careful annotation of images such as CT scans and x-rays along with MRI’s etcetera depending upon how advanced each system needs be.
Driver Clocks And Longevity — Dissecting True Functional “Drivers” Of Aging Phenotypes — Dr. Daniel Ives Ph.D., Founder and CEO — Shift Bioscience Ltd.
Dr. Daniel Ives, Ph.D. is Founder and CEO of Shift Bioscience Ltd. (https://shiftbioscience.com), a biotech company making drugs for cellular rejuvenation in humans through the application of machine-learning ‘driver’ clocks to cellular reprogramming, and is the scientific founder who first discovered the gene shifting targets upon which the Shift drug discovery platform is based.
Dr. Ives graduated from Imperial College with a degree in biochemistry and gained his PhD in 2013 working at the MRC Mitochondrial Biology Unit in Cambridge. He carried out his post-doctoral studies under Ian Holt at the National Institute of Medical Research in Mill Hill, now part of the Crick Institute, pursuing damage-removal strategies for mitochondrial DNA mutations.
In 2016 Dr. Ives left the Crick Institute and founded Shift Bioscience to commercialize mitochondrial targeted drugs for age linked diseases, incorporating novel ageing biomarkers technologies, CRISPR screens, and other tools to dissect true functional ‘drivers’ of ageing phenotypes.
A licensed drug normally used to treat abnormal levels of fatty substances in the blood could reduce infection caused by the SARS-CoV-2 virus by up to 70 percent, reveals a study in the laboratory by an international collaboration of researchers.
The research team, led by the University of Birmingham and Keele University in the UK and the San Raffaele Scientific Institute in Italy, has demonstrated that fenofibrate and its active form (fenofibric acid) can significantly reduce SARS-COV-2 infection in human cells in the laboratory. Importantly, reduction of infection was obtained using concentrations of the drug which are safe and achievable using the standard clinical dose of fenofibrate. Fenofibrate, which is approved for use by most countries in the world including the US Food and Drug Administration (FDA) and the UK’s National Institute for Health and Care Excellence (NICE), is an oral drug currently used to treat conditions such as high levels of cholesterol and lipids (fatty substances) in the blood.
The team is now calling for clinical trials to test the drug in hospitalized COVID-19 patients, to be carried out in addition to two clinical trials also currently underway in such patients in research being led by the Hospital of the University of Pennsylvania in the US and Hebrew University of Jerusalem in Israel.
The Pfizer coronavirus vaccine may be linked to a form of eye inflammation called uveitis, according to a multicenter Israeli study led by Prof. Zohar Habot-Wilner from Tel Aviv’s Sourasky Medical Center.
The research was conducted at Rambam Health Care Campus, Galilee Medical Center, Shaare Zedek Medical Center, Sheba Medical Center in Tel Hashomer, Kaplan Medical Center and Sourasky. It was accepted for publication by the peer-reviewed ophthalmology journal Retina.
Habot-Wilner, head of the Uveitis Service at the hospital, found that 21 people (23 eyes) who had received two shots of the Pfizer vaccine developed uveitis within one to 14 days after receiving their first shot or within one day to one month after the second.
Researchers remain perplexed as to why some patients infected with SARS-CoV-2, the virus responsible for COVID-19, remain asymptomatic while other patients develop severe disease symptoms. This question is once again at the front of mind as the Delta variant spreads across the country. In a new retrospective study, researchers at the Medical University of South Carolina (MUSC) discovered a specific and sensitive biomarker in blood samples that predicts which patients will develop COVID-19 symptoms. Their results, published online on July 9 in Scientific Reports, show that reduced levels of a specific lipid, sphingosine, are significantly associated with developing COVID-19 symptoms. Conversely, elevated levels of sphingosine, as well as a protein involved in its production, acid ceramidase (AC), are associated with asymptomatic infections.
“We developed this project at a time when there wasn’t a successful vaccine,” said Besim Ogretmen, Ph.D., director of the Lipidomics Shared Resource at Hollings Cancer Center and leader of the Hollings Developmental Cancer Therapeutics Research Program. “We wanted to contribute to the field and know which patients who were exposed to this virus would be symptomatic versus asymptomatic.”
Over the past 16 months several waves of SARS-CoV-2 infections in the U.S. have resulted in more than 35 million cases and almost 630000 deaths. Despite the development of multiple safe and effective vaccines, we are currently experiencing another wave of infections.
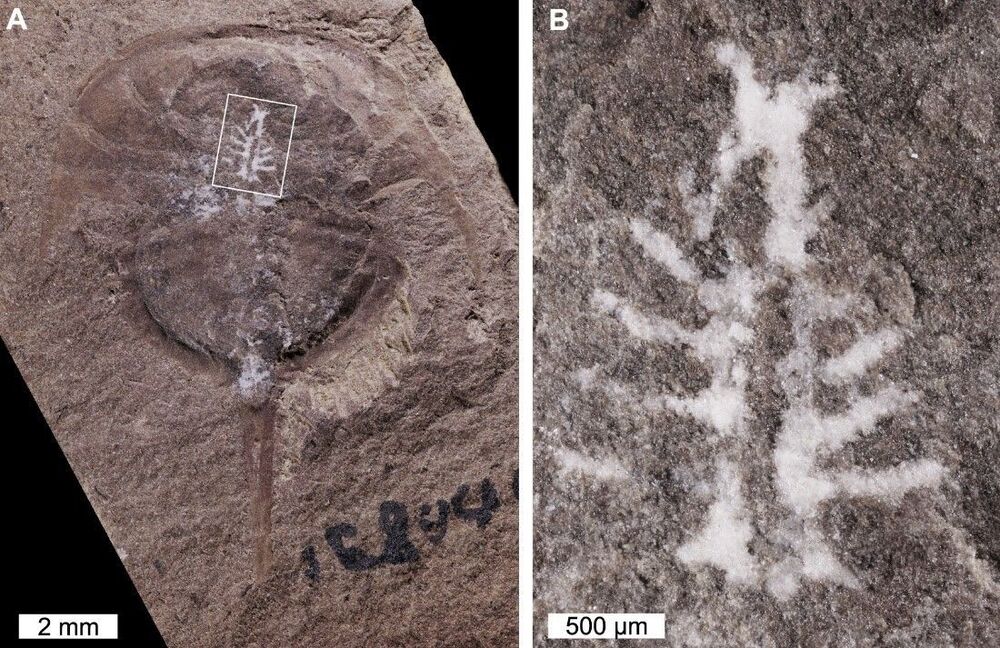
Researchers have discovered one of the oldest and best-preserved brains in the fossil record. A 310-million-year-old horseshoe crab was found with its complete brain intact, thanks to a previously unknown preservation method.
The majority of our knowledge of ancient creatures comes from bones – soft tissues don’t fossilize very well. Some mechanisms are better than others at preserving these fragile tissues though, most famously amber. Scientists can then scan amber-encased creatures to image their brains and other organs.
But that record only goes back so far. The oldest amber inclusions date back about 230 million years ago, to the Triassic period. Burgess Shale-type deposits, however, extend as far back as 520 million years ago, to the early Cambrian. These mudstone deposits can also preserve soft tissues as carbon films – most commonly the gut, but on rare occasions imprints of parts of the nervous system can be found.
Upon an otherwise unruly landscape of choppy sea and craggy peaks, the salmon farms that dot many of Norway’s remote fjords impose a neat geometry. The circular pens are placid on the surface, but hold thousands of churning fish, separated by only a net from their wild counterparts. And that is precisely the conundrum. Although the pens help ensure the salmon’s welfare by mimicking the fish’s natural habitat, they also sometimes allow fish to escape, a problem for both the farm and the environment.
In an attempt to prevent escaped fish from interbreeding with their wild counterparts and threatening the latter’s genetic diversity, molecular biologist Anna Wargelius and her team at the Institute of Marine Research in Norway have spent years working on ways to induce sterility in Atlantic salmon. Farmed salmon that cannot reproduce, after all, pose no threat to the gene pool of wild stocks, and Wargelius has successfully developed a technique that uses the gene-editing technology Crispr to prevent the development of the cells that would otherwise generate functioning sex organs.
In fact, Wargelius’ team was a little too successful. To be financially viable, commercial fish farms need at least some of their stock to reproduce. So the scientists went a step further, developing a method of temporarily reversing the modification they had already made. They’ve created what they call “sterile parents.”