If you needed a heart transplant or suffered from cardiovascular disease, would you consider replacing your biological heart for an artificial one? How many of you with healthy hearts would want the transplant?
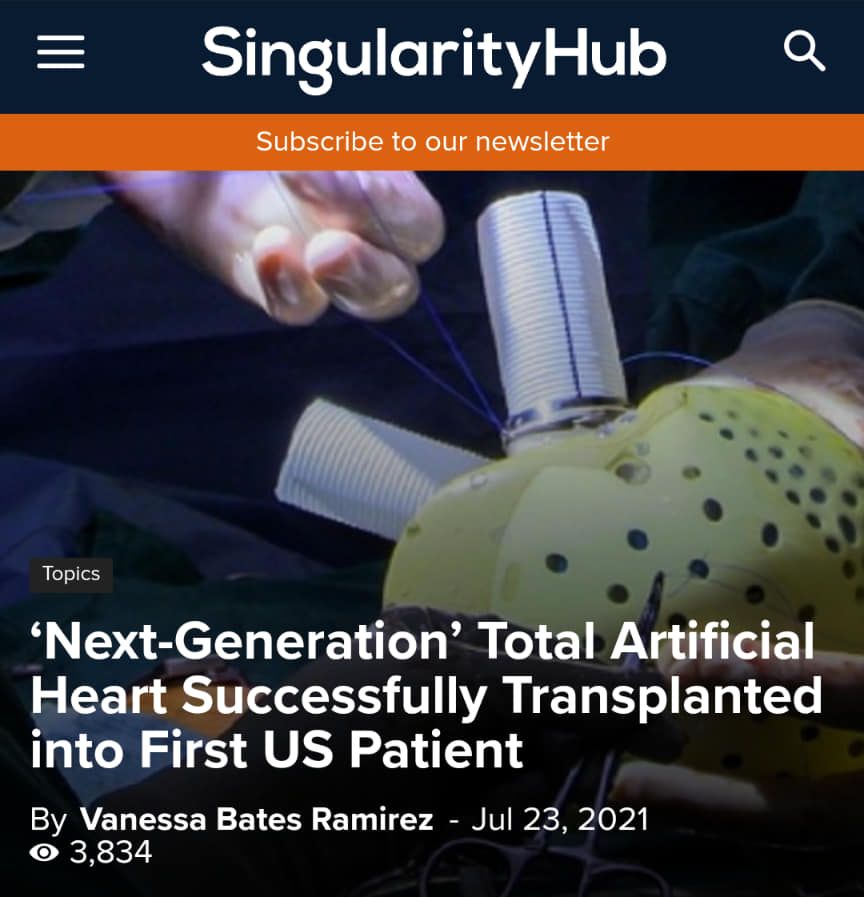




I think I posted about the work in Texas, but here is more work.
Israeli and American researchers have discovered a nanobody cocktail that could neutralize coronavirus, including the Delta mutation.
Nanobodies are single domain antibodies derived from llamas — or other members of the camel family.
The discovery of the cocktail and their effectiveness in combating coronavirus was published in the peer-reviewed journal Nature Communications.
“If we can produce an innovative drug through the cocktail, it will be a life-saving treatment — if given early in the disease,” according to Hebrew University School of Engineering and Computer Science Dr. Dina Schneidman-Duhovy, who helped lead the study with University of Pittsburgh researcher Dr. Yi Shi.
A team of Israeli and American researchers have found a combination of antibodies derived from llamas that may be effective in treating the coronavirus.
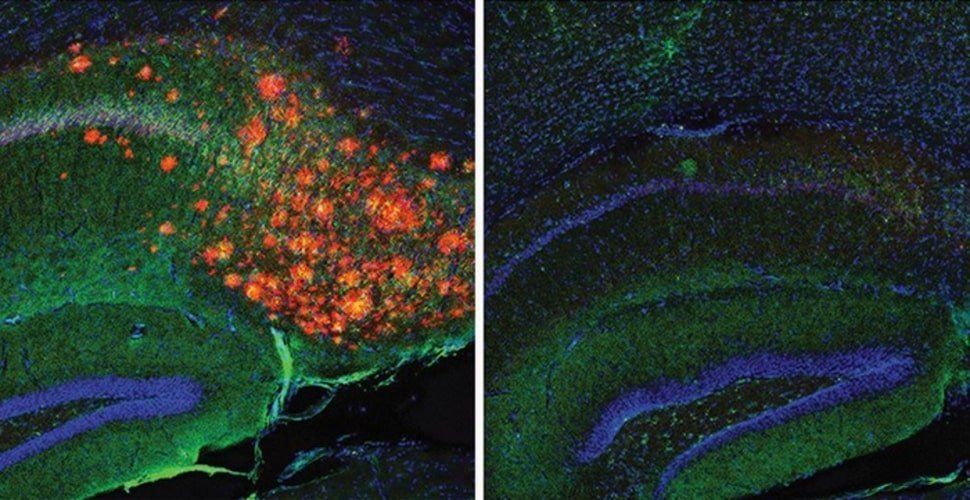
“We showed that cholesterol is acting essentially as a signal in neurons that determines how much Aβ gets made—and thus it should be unsurprising that apoE, which carries the cholesterol to neurons, influences Alzheimer’s risk,” says study co-senior author Scott Hansen, PhD, an associate professor in the Department of Molecular Medicine at Scripps Research, Florida.
Summary: A new advanced imaging technique shows how cholesterol regulates the production of Alzheimer’s associated amyloid beta proteins in astrocytes.
Source: Scripps Research Institute
A team co-led by scientists at Scripps Research has used advanced imaging methods to reveal how the production of the Alzheimer’s-associated protein amyloid beta (Aβ) in the brain is tightly regulated by cholesterol.
Appearing on line Thursday ahead of print in the Aug. 17 issue of the Proceedings of the National Academy of Sciences (PNAS), the scientists’ work advances understanding of how Alzheimer’s disease develops and underscores the long-underappreciated role of brain cholesterol.
These researchers recently published a study on VDAC2, a protein that helps regulate calcium signaling within heart cells. Blockage of the signals causes severe impairment of heart cell contraction, making it harder for the organ to push blood through the body.
Taking away this protein made heart function sharply decline in laboratory mice, eventually leading to their death, while reintroduction of VDAC2 reversed many of the effects of heart failure. An experimental drug called efsevin was able to produce similar effects in other mice with heart failure.
With the epidemic of heart failure exacerbating the pandemic of COVID-19, the discovery by University of Utah researchers in Salt Lake City of a protein in heart cells brings the potential for a method to improve heart function in patients.
Bionic arms used to cost $80,000. Now, a young engineer has lowered the cost by over 90%.
Subscribe here: https://freeth.ink/youtube-subscribe-toc.
Unlimited Tomorrow is pioneering a new age in prosthetics with its 3D-printed robotic arms. Founded in 2,014 by Easton LaChapelle when he was just 18 years old, the company is poised to become a leader in the prosthetic arm industry. Their True Limb device costs less than $8,000 and it’s even cheaper for children, priced at about $4,000.
True Limb is both functional and realistic-looking, serving as a mirror image of the amputee’s opposing limb, even down to the fingertips. And while the prosthetic arm is 60–90% cheaper than traditional prosthetics, many users say it’s far superior to market alternatives. What’s the secret? Unlimited Tomorrow uses a totally remote, custom process that cuts out middlemen to produce prosthetics completely in-house.
For the 40-million worldwide amputees in need of prosthetic limbs, this remote, personalized, and affordable process for fitting prosthetics means hope for a better future.
See the full article on bionic arms here: https://www.freethink.com/series/challengers/prosthetic-arm.
This is interesting. 😃
A new discovery in rats shows that the brain responds differently in immersive virtual reality environments versus the real world. The finding could help scientists understand how the brain brings together sensory information from different sources to create a cohesive picture of the world around us. It could also pave the way for “virtual reality therapy” for learning and memory-related disorders ranging including ADHD, Autism, Alzheimer’s disease, epilepsy and depression.
Mayank Mehta, PhD, is the head of W. M. Keck Center for Neurophysics and a professor in the departments of physics, neurology, and electrical and computer engineering at UCLA. His laboratory studies a brain region called the hippocampus, which is a primary driver of learning and memory, including spatial navigation. To understand its role in learning and memory, the hippocampus has been extensively studied in rats as they perform spatial navigation tasks.
When rats walk around, neurons in this part of the brain synchronize their electrical activity at a rate of 8 pulses per second, or 8 Hz. This is a type of brain wave known as the “theta rhythm,” and it was discovered more than six decades ago.
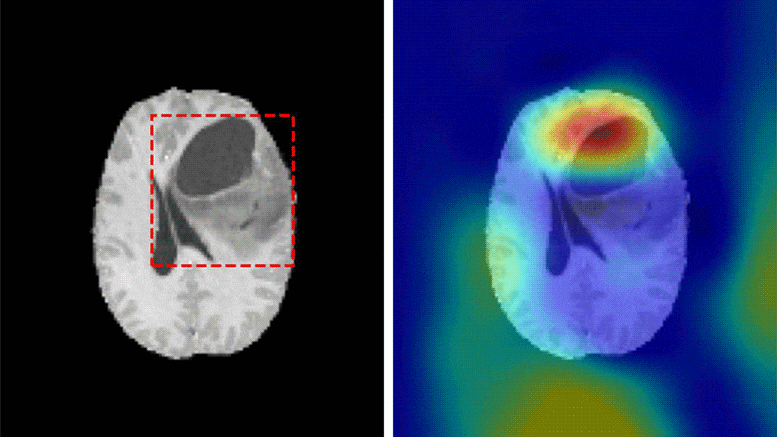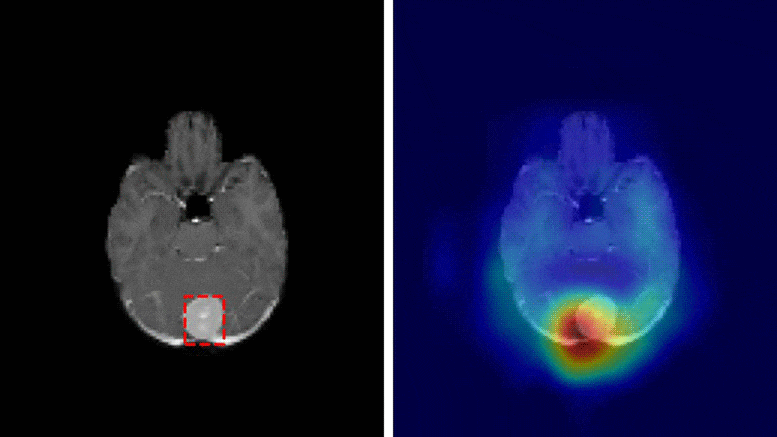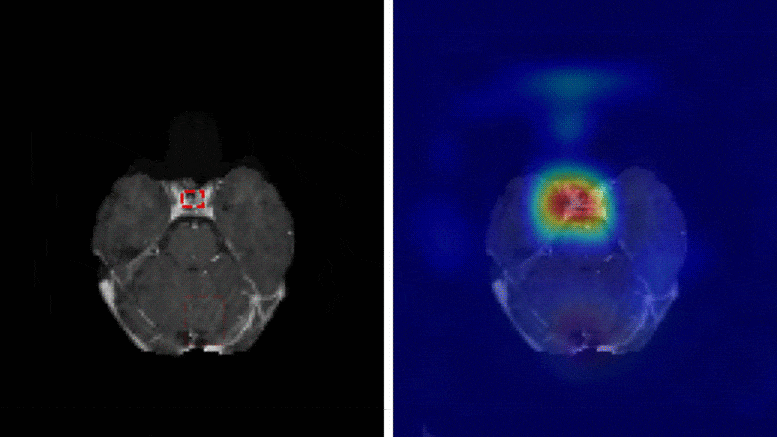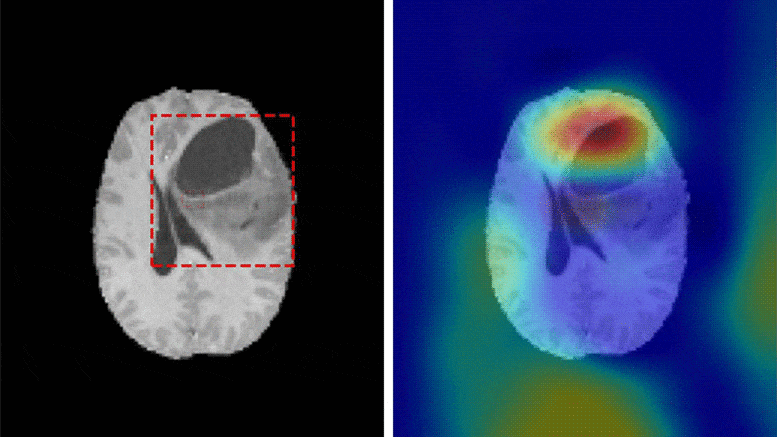
A team of researchers at Washington University School of Medicine have developed a deep learning model that is capable of classifying a brain tumor as one of six common types using a single 3D MRI scan, according to a study published in Radiology: Artificial Intelligence.
“This is the first study to address the most common intracranial tumors and to directly determine the tumor class or the absence of tumor from a 3D MRI volume,” said Satrajit Chakrabarty, M.S., a doctoral student under the direction of Aristeidis Sotiras, Ph.D., and Daniel Marcus, Ph.D., in Mallinckrodt Institute of Radiology’s Computational Imaging Lab at Washington University School of Medicine in St. Louis, Missouri.
The six most common intracranial tumor types are high-grade glioma, low-grade glioma, brain metastases, meningioma, pituitary adenoma and acoustic neuroma. Each was documented through histopathology, which requires surgically removing tissue from the site of a suspected cancer and examining it under a microscope.
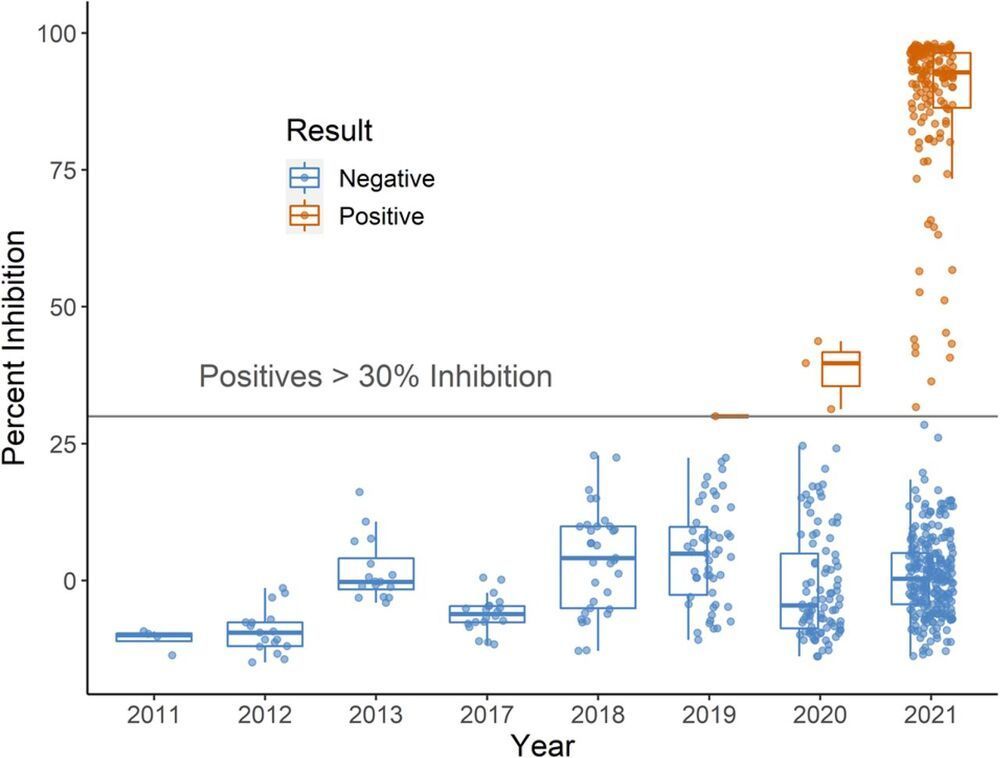
Widespread human SARS-CoV-2 infections combined with human-wildlife interactions create the potential for reverse zoonosis from humans to wildlife. We targeted white-tailed deer (Odocoileus virginianus) for serosurveillance based on evidence these deer have ACE2 receptors with high affinity for SARS-CoV-2, are permissive to infection, exhibit sustained viral shedding, can transmit to conspecifics, and can be abundant near urban centers. We evaluated 624 pre-and post-pandemic serum samples from wild deer from four U.S. states for SARS-CoV-2 exposure. Antibodies were detected in 152 samples (40%) from 2,021 using a surrogate virus neutralization test. A subset of samples was tested using a SARS-CoV-2 virus neutralization test with high concordance between tests. These data suggest white-tailed deer in the populations assessed have been exposed to SARS-CoV-2.
One-Sentence Summary Antibodies to SARS-CoV-2 were detected in 40% of wild white-tailed deer sampled from four U.S. states in 2021.
SARS-CoV-2, the virus that causes COVID-19 in humans, can infect multiple domestic and wild animal species (1 – 7). Thus, the possibility exists for the emergence of new animal reservoirs of SARS-CoV-2, each with unique potential to maintain, disseminate, and drive novel evolution of this virus. Of particular concern are wildlife species that are both abundant and live in close association with human populations (5).