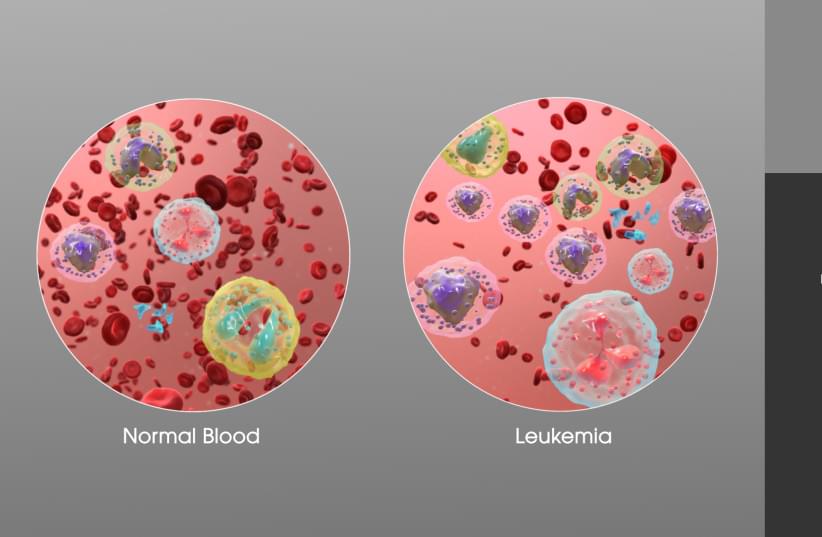
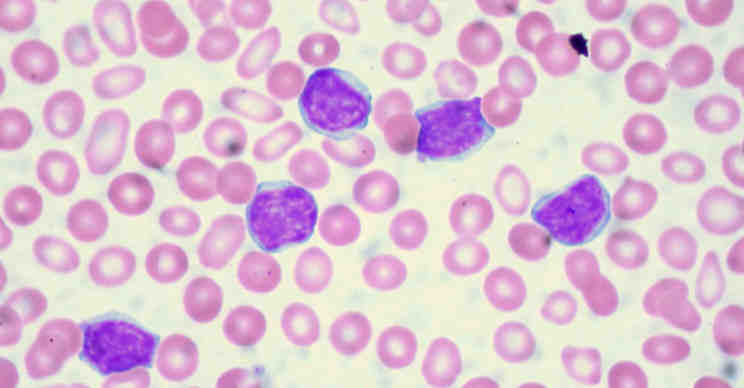
In cancer, healthy cells turn into malignant ones with very different characteristics, such as the ability to divide in an uncontrolled manner. In recent decades, much research has uncovered various molecular alterations responsible for this conversion from healthy to tumor tissue. But until now, scientists have known very little about the opposite process – reversing a cancer cell, turning it into a physiological, noncancerous one, and what factors might mediate this process.
“We know that one strategy that human tumors have to dodge the effectiveness of drugs is to change their appearance, becoming another similar cancer but insensitive to the drug used,” the team said. “For example, leukemias of the lymphoid lineage are switched to the myeloid strain to escape treatment.”
With this idea in mind, they wanted to know more about the molecular pathways involved in this cellular transformation. They studied an in vitro model (experiment performed outside of a living organism, usually in a test tube or petri dish) in which leukemia cells can be forced to turn into a type of harmless immune cells called macrophages.