U.S. regulators have approved a new purple tomato, genetically engineered to be packed with antioxidants and anthocyanins. The fruit will go on sale in 2023.


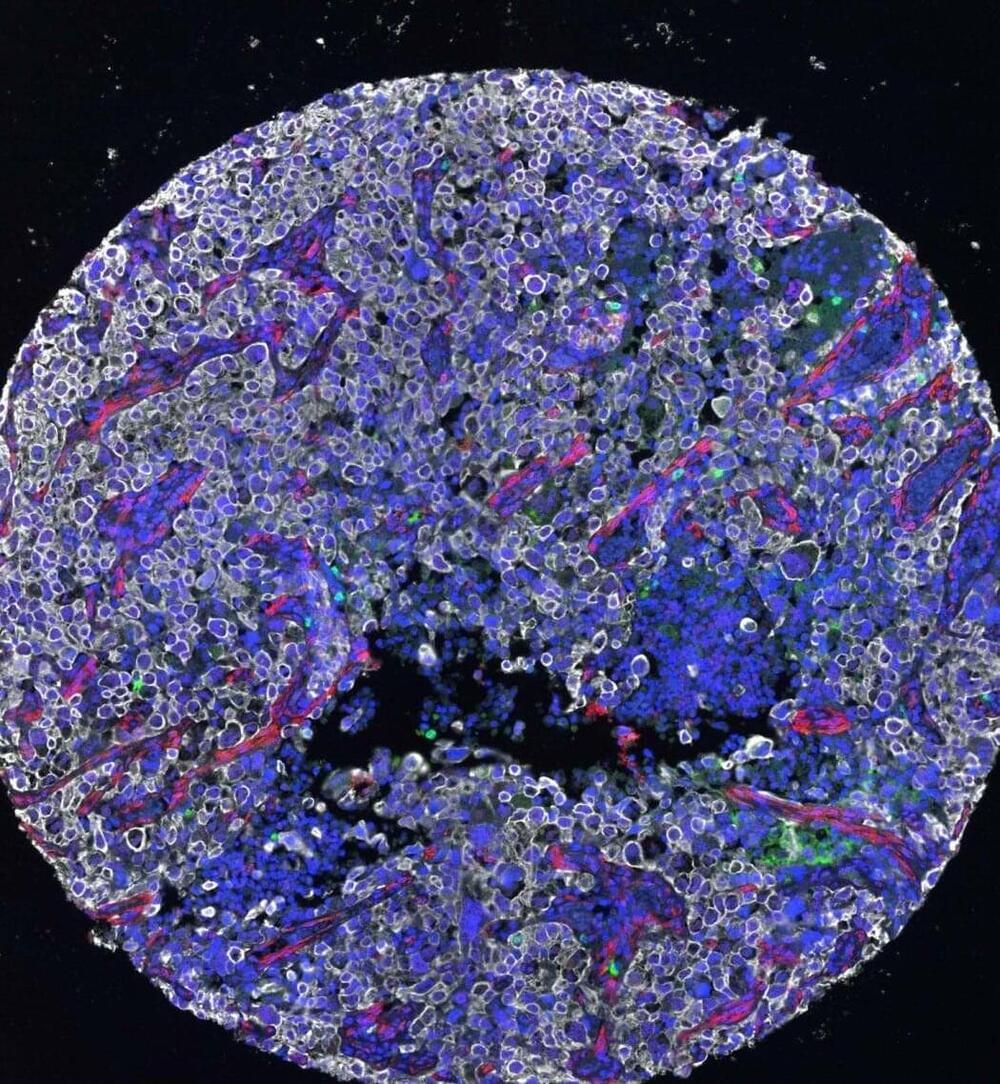
Tumor cells are notoriously good at evading the human immune system; they put up physical walls, wear disguises and handcuff the immune system with molecular tricks. Now, UC San Francisco researchers have developed a drug that overcomes some of these barriers, marking cancer cells for destruction by the immune system.
The new therapy, described in Cancer Cell, pulls a mutated version of the protein KRAS to the surface of cancer cells, where the drug-KRAS complex acts as an “eat me” flag. Then, an immunotherapy can coax the immune system to effectively eliminate all cells bearing this flag.
“The immune system already has the potential to recognize mutated KRAS, but it usually can’t find it very well. When we put this marker on the protein, it becomes much easier for the immune system,” said UCSF chemist and Howard Hughes Medical Institute Investigator Kevan Shokat, Ph.D., who also helped lead the new work.
Being able to decode brainwaves could help patients who have lost the ability to speak to communicate again, and could ultimately provide novel ways for humans to interact with computers. Now Meta researchers have shown they can tell what words someone is hearing using recordings from non-invasive brain scans.
Our ability to probe human brain activity has improved significantly in recent decades as scientists have developed a variety of brain-computer interface (BCI) technologies that can provide a window into our thoughts and intentions.
The most impressive results have come from invasive recording devices, which implant electrodes directly into the brain’s gray matter, combined with AI that can learn to interpret brain signals. In recent years, this has made it possible to decode complete sentences from someone’s neural activity with 97 percent accuracy, and translate attempted handwriting movements directly into text at speeds comparable to texting.
(Medical Xpress)—A team of researchers at the Max Planck Institute has found what they believe is the DNA mutation that led to a change in function of a gene in humans that sparked the growth of a larger neocortex. In their paper published in the journal Science Advances, the team describes how they engineered a gene found only in humans, Denisovans and Neanderthals to look like a precursor to reveal its neuroproliferative effect.
A year ago, another team of researchers found the human gene that most in the field believe was a major factor in allowing the human brain to grow bigger, allowing for more complex processing. In this new effort, the researchers have found what they believe was the DNA change that arose in that gene.
To pinpoint that change, the researchers engineered the unique ARHGAP11B gene to make it more similar to the ARHGAP11A gene, which researchers believe was a predecessor gene—they swapped a single nucleotide (out of 55 possibilities) for another and in so doing, found the ARHGAP11B gene lost its neuroproliferative abilities. This, the team claims, shows that it was a single mutation that allowed humans to grow bigger brains. Such a mutation, they note, was not likely due to natural selection, but was more likely a simple mistake that occurred as a brain cell was splitting. Because it conferred an advantage (the ability to grow higher than normal amounts of brain cells) the mutation was retained through subsequent generations. They also point out that such a mutation would have resulted specifically in a larger neocortex—a portion of the cortex that has been associated with hearing and sight.

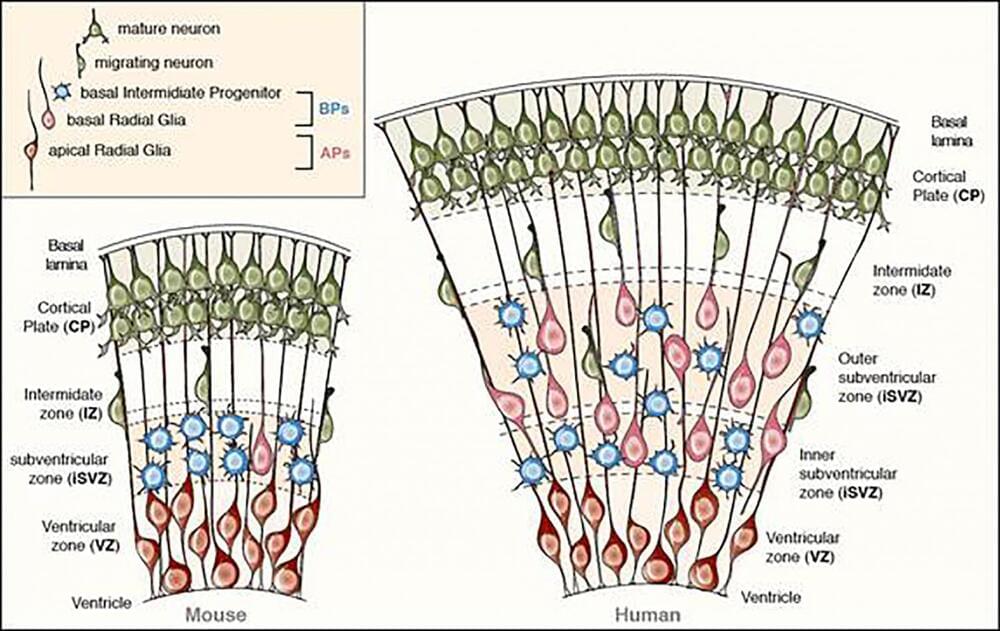
When the right gene is expressed in the right manner in the right population of stem cells, the developing mouse brain can exhibit primate-like features. In a paper publishing August 7th in the Open Access journal PLOS Biology, researchers at the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) succeeded in mimicking the sustained expression of the transcription factor Pax6 as seen in the developing human brain, in mouse cortical progenitor cells. This altered the behavior of these cells to one that is akin to that of progenitors in the developing primate neocortex. Consequently, the mouse progenitors generated more neurons — a prerequisite for a bigger brain.
The neocortex consists of different types of progenitors, but one particular class, the basal progenitors, behave differently in small-brained animals such as mice than in large-brained animals such as humans. In humans, basal progenitors can undergo multiple rounds of cell division, thereby substantially increasing neuron number and ultimately the size of the neocortex. In mice, these progenitors typically undergo only one round of cell division, thus limiting the number of neurons produced. A potential cause underlying this difference in the proliferative capacity of basal progenitors could be the differential expression of Pax6 between species. Mouse basal progenitors, in contrast to human, do not express Pax6. “We were very curious to see what would happen if we were to change the expression pattern of Pax6 in developing mouse brain to mimic that observed in large-brained animals”, says Fong Kuan Wong, a PhD student in the lab of Wieland Huttner and first author of the study.
To this end, another PhD student in the lab, Ji-Feng Fei, generated a novel transgenic mouse line. This line provided the basis for altering the expression of Pax6 in the cortical stem cell lineage such that it would be sustained in basal progenitors. The researchers then introduced the Pax6 gene into the stem cells of these mice. Strikingly, sustaining Pax6 expression in mouse basal progenitors increased their capacity to undergo multiple rounds of cell division, as typically observed in primates. This not only expanded the size of the basal progenitor population in a way somewhat reminiscent to what is seen in large-brained animals. It also resulted in an increase in cortical neurons, notably those in the top layer, another characteristic feature of an expanded neocortex.
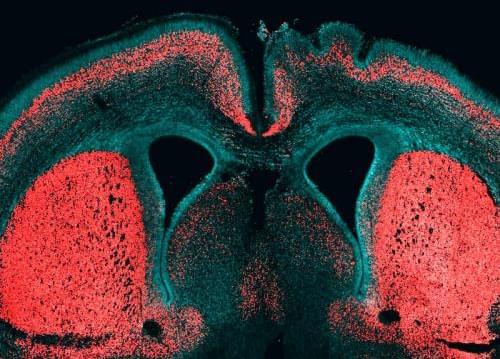
About 99 percent of human genes are shared with chimpanzees. Only the small remainder sets us apart. However, we have one important difference: The brain of humans is three times as big as the chimpanzee brain.
During evolution our genome must have changed in order to trigger such brain growth. Wieland Huttner, Director and Research Group Leader a the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG), and his team identified for the first time a gene that is only present in humans and contributes to the reproduction of basal brain stem cells, triggering a folding of the neocortex. The researchers isolated different subpopulations of human brain stem cells and precisely identified, which genes are active in which cell type. In doing so, they noticed the gene ARHGAP11B: it is only found in humans and in our closest relatives, the Neanderthals and Denisova-Humans, but not in chimpanzees. This gene manages to trigger brain stem cells to form a bigger pool of stem cells. In that way, during brain development more neurons can arise and the cerebrum can expand. The cerebrum is responsible for cognitive functions like speaking and thinking.
Wieland Huttner’s researchers developed a method that isolates and identifies special subpopulations of brain stem cells from the developing human cerebrum. No one has managed to do this so far. The scientists first isolated different stem and progenitor cell types from fetal mice and human cerebrum tissue. In contrast to the big and folded human brain, the brain of mice is small and smooth. After the isolation, the researchers compared the genes that are active in the various cell types and were able to identify 56 genes that are only present in humans and which play a role in brain development. “We noticed that the gene ARHGAP11B is especially active in basal brain stem cells. These cells are really important for the expansion of the neocortex during evolution,” says Marta Florio, PhD student in Wieland Huttner’s lab, who carried out the main part of the study.

During fetal development of the mammalian brain, the cerebral cortex undergoes a marked expansion in surface area in some species, which is accommodated by folding of the tissue in species with most expanded neuron numbers and surface area. Researchers have now identified a key regulator of this crucial process.
Different regions of the mammalian brain are devoted to the performance of specific tasks. This in turn imposes particular demands on their development and structural organization. In the vertebrate forebrain, for instance, the cerebral cortex – which is responsible for cognitive functions – is remarkably expanded and extensively folded exclusively in mammalian species. The greater the degree of folding and the more furrows present, the larger is the surface area available for reception and processing of neural information. In humans, the exterior of the developing brain remains smooth until about the sixth month of gestation. Only then do superficial folds begin to appear and ultimately dominate the entire brain in humans. Conversely mice, for example, have a much smaller and smooth cerebral cortex.
“The mechanisms that control the expansion and folding of the brain during fetal development have so far been mysterious,” says Professor Magdalena Götz, a professor at the Institute of Physiology at LMU and Director of the Institute for Stem Cell Research at the Helmholtz Center Munich. Götz and her team have now pinpointed a major player involved in the molecular process that drives cortical expansion in the mouse. They were able to show that a novel nuclear protein called Trnp1 triggers the enormous increase in the numbers of nerve cells which forces the cortex to undergo a complex series of folds. Indeed, although the normal mouse brain has a smooth appearance, dynamic regulation of Trnp1 results in activating all necessary processes for the formation of a much enlarged and folded cerebral cortex.

To an untrained observer, the electrical storm that takes place over the brain’s neural network seems a chaotic flurry of activity. But as neuroscientists understand it, the millions of neurons are actually engaged in a sort of tightly choreographed dance, a tango of excitatory and inhibitory neurons. How is this precise balance that makes normal function possible achieved during development? And how does it go wrong in diseases like epilepsy when brain activity goes out of control?
Focusing on the cerebral cortex, the part of the brain controlling thought, sensory awareness, and motor function, a group of Harvard Stem Cell Institute (HSCI) researchers in the Department of Stem Cell and Regenerative Biology (SCRB), led by Assistant Professor Paola Arlotta, has discovered that excitatory neurons control the positioning of inhibitory neurons in a process that is critically important for generating balanced circuitry and proper cortical response.
Professor Takao Hensch, a collaborator on the study in the Harvard Center for Brain Science, Department of Molecular & Cellular Biology (MCB), had previously shown that the maturation of this circuit balance triggers critical periods of brain development. Certain inhibitory cells appear particularly vulnerable to genetic or environmental factors in early life, contributing to mental illness, such as schizophrenia or autism spectrum disorders.
“We put nanotubes inside of bacteria,” says Professor Ardemis Boghossian at EPFL’s School of Basic Sciences. “That doesn’t sound very exciting on the surface, but it’s actually a big deal. Researchers have been putting nanotubes in mammalian cells that use mechanisms like endocytosis, that are specific to those kinds of cells. Bacteria, on the other hand, don’t have these mechanisms and face additional challenges in getting particles through their tough exterior. Despite these barriers, we’ve managed to do it, and this has very exciting implications in terms of applications.”
Boghossian’s research focuses on interfacing artificial nanomaterials with biological constructs, including living cells. The resulting “nanobionic” technologies combine the advantages of both the living and non-living worlds. For years, her group has worked on the nanomaterial applications of single-walled carbon nanotubes (SWCNTs), tubes of carbon atoms with fascinating mechanical and optical properties.
These properties make SWCNTs ideal for many novel applications in the field of nanobiotechnology. For example, SWCNTs have been placed inside mammalian cells to monitor their metabolisms using near-infrared imaging. The insertion of SWCNTs in mammalian cells has also led to new technologies for delivering therapeutic drugs to their intracellular targets, while in plant cells they have been used for genome editing. SWCNTs have also been implanted in living mice to demonstrate their ability to image biological tissue deep inside the body.