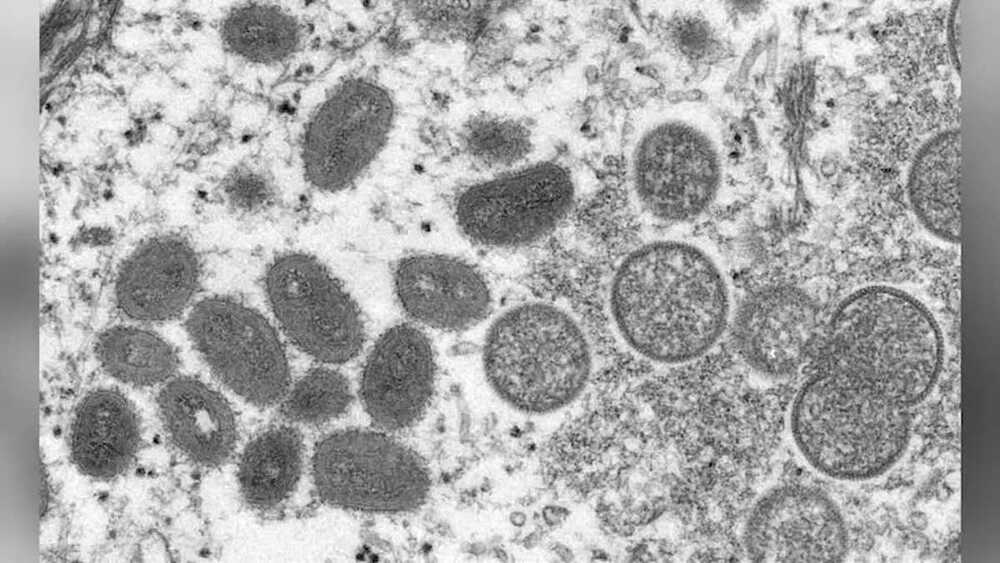
A Massachusetts resident has tested positive for monkeypox, health officials confirmed Wednesday, making it the first case of the rare virus detected in the United States this year.
According to a release from the Massachusetts Department of Public Health, the patient is an adult male who recently traveled to Canada. The department completed initial testing Tuesday and was confirmed by the Centers for Disease Control and Prevention.
“The case poses no risk to the public, and the individual is hospitalized and in good condition,” MDPH stated in a press release. “DPH is working closely with the CDC, relevant local boards of health, and the patient’s health care providers to identify individuals who may have been in contact with the patient while he was infectious.”