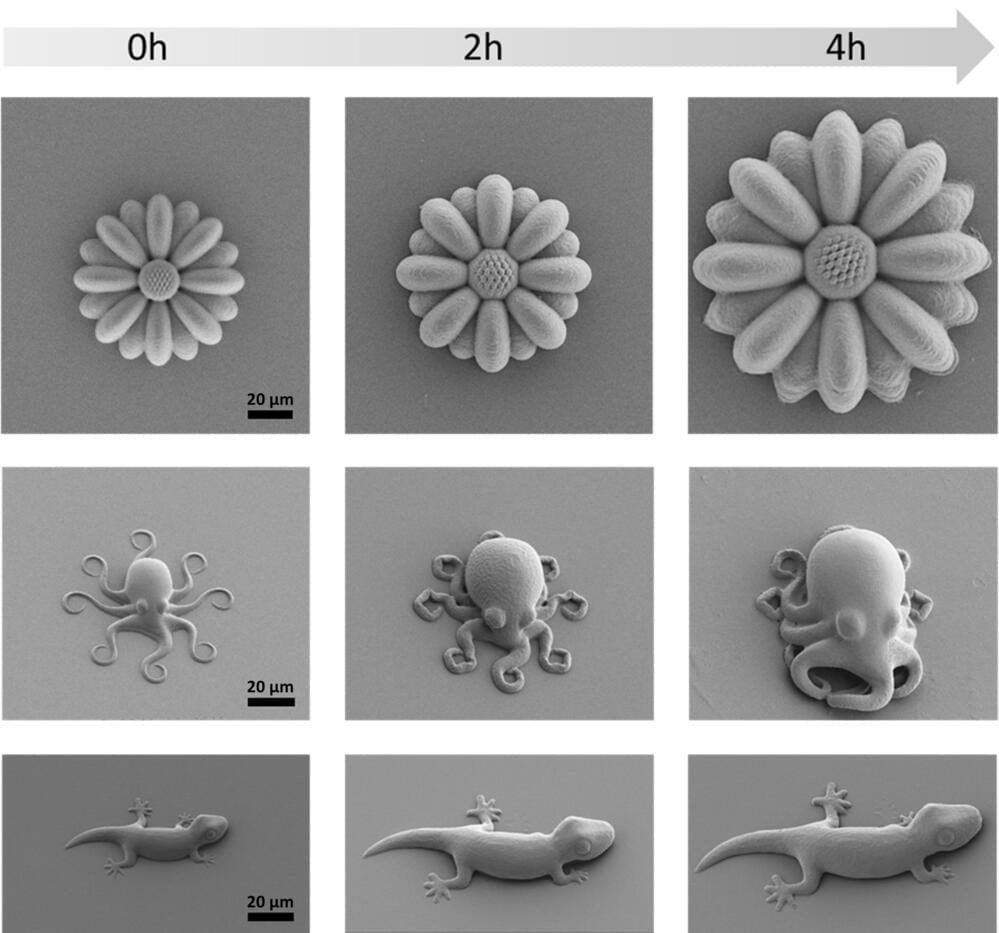
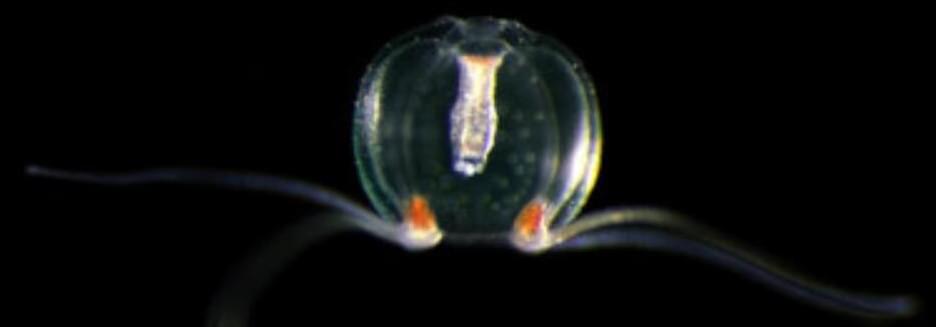
Although just cute little creatures at first glance, the microscopic geckos and octopuses fabricated by 3D laser printing in the molecular engineering labs at Heidelberg University could open up new opportunities in fields such as microrobotics or biomedicine.
The printed microstructures are made from novel materials —known as smart polymers—whose size and mechanical properties can be tuned on demand and with high precision. These “life-like” 3D microstructures were developed in the framework of the “3D Matter Made to Order” (3DMM2O) Cluster of Excellence, a collaboration between Ruperto Carola and the Karlsruhe Institute of Technology (KIT).
“Manufacturing programmable materials whose mechanical properties can be adapted on demand is highly desired for many applications,” states Junior Professor Dr. Eva Blasco, group leader at the Institute of Organic Chemistry and the Institute for Molecular Systems Engineering and Advanced Materials of Heidelberg University.