Talking about some of the ideas and philosophy surrounding life extension technologies. Our own psychology and coping mechanisms that view death as a good thing. The same way we used to see some diseases as a part of a gods plan. As soon as we cured these diseases, somehow they were not a part of gods plan anymore. The same will happen with aging and death, and that is just a matter of time. Picking apart some of the ways of thinking that suggest a longer life would be a boring or bad thing. We live for all of the pleasant and amazing experiences that we can have in the world, what else could possibly matter more. The end and absence of meaning (death) does not give life meaning. It is life that gives life meaning.
Category: biotech/medical – Page 1,346
Chester the AI Radiology Assistant
In order to bridge the gap between AI researchers and medical professionals we developed a very accessible free prototype system which can be used by medical professionals to understand the reality of Deep Learning tools for chest X-ray diagnostics.
‘Mind-Reading’ Technology Translates Brainwaves into Photos
“I believe we can train the algorithm not only to picture accurately a face you’re looking at, but also any face you imagine vividly, such as your mother’s,” explains Dado.
“By developing this technology, it would be fascinating to decode and recreate subjective experiences, perhaps even your dreams,” Dado says. “Such technological knowledge could also be incorporated into clinical applications such as communicating with patients who are locked within deep comas.”
Dado’s work is focused on using the technology to help restore vision in people who, through disease or accident, have become blind, reports the Mail Online.
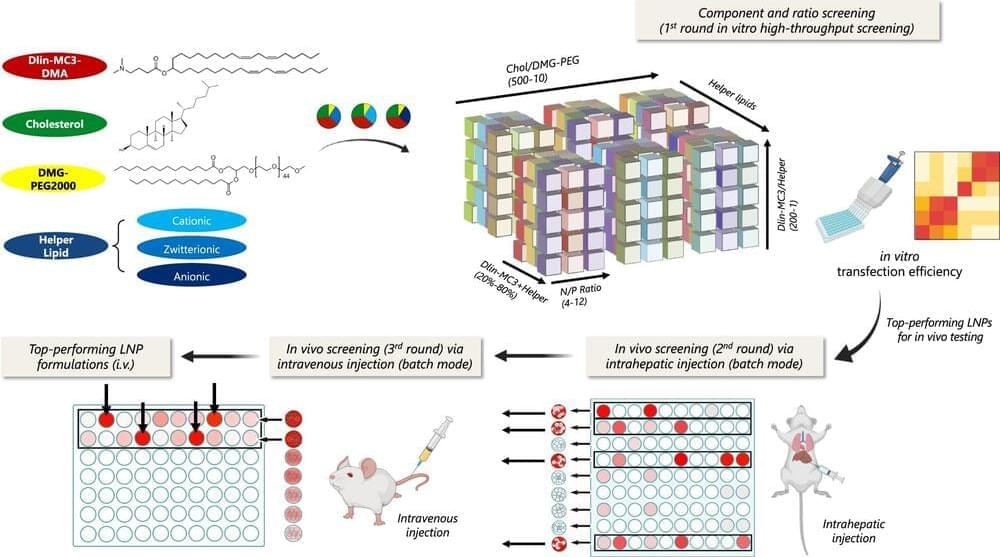
New platform could make gene medicine delivery easier and more affordable
The success of COVID-19 vaccines is a great example of gene medicine’s tremendous potential to prevent viral infections. One reason for the vaccines’ success is their use of lipid nanoparticles, or LNPs, to carry delicate messenger RNA to cells to generate and boost immunity. LNPs—tiny fat particles—have become increasingly popular as a carrier to deliver various gene-based medicines to cells, but their use is complicated because each LNP must be tailored specifically for the therapeutic payload it carries.
A team led by Hai-Quan Mao, a Johns Hopkins materials scientist, has created a platform that shows promise to speed up the LNP design process and make it more affordable. The new approach also can be adapted to other gene therapies.
“In a nutshell, what we have done is creating a method that screens lipid nanoparticle components and their proportions to quickly help identify and create the optimal design for use with various therapeutic genes,” said Mao, director of the Institute for NanoBioTechnology at Johns Hopkins Whiting School of Engineering and professor in the departments of Materials Science and Engineering and Biomedical Engineering.
New research on the risks of lead exposure from bullets used in big game hunting
The lead in some bullets used for hunting deer, moose, and elk is toxic to the humans who eat the harvested meat and to scavenger animals that feast on remains left in the field.
A team of researchers from the Canadian Light Source at the University of Saskatchewan (USask) and the College of Medicine at USask has for the first time used synchrotron imaging to study both the size and spread of bullet fragments in big game shot by hunters. Their findings were published today in PLOS ONE.
Like a scene right out of the hit television series CSI, the research team fired bullets into blocks of ballistic gelatin—the same material used by law enforcement agencies for ballistic testing—and examined the resulting fragments using synchrotron imaging.
Researchers engineer novel material capable of ‘thinking’
Someone taps your shoulder. The organized touch receptors in your skin send a message to your brain, which processes the information and directs you to look left, in the direction of the tap. Now, Penn State and U.S. Air Force researchers have harnessed this processing of mechanical information and integrated it into engineered materials that “think”.
The work, published today in Nature, hinges on a novel, reconfigurable alternative to integrated circuits. Integrated circuits are typically composed of multiple electronic components housed on a single semiconductor material, usually silicon, and they run all types of modern electronics, including phones, cars and robots. Integrated circuits are scientists’ realization of information processing similar to the brain’s role in the human body. According to principal investigator Ryan Harne, James F. Will Career Development Associate Professor of Mechanical Engineering at Penn State, integrated circuits are the core constituent needed for scalable computing of signals and information but have never before been realized by scientists in any composition other than silicon semiconductors.
His team’s discovery revealed the opportunity for nearly any material around us to act like its own integrated circuit: being able to “think” about what’s happening around it.
Supercomputing center dataset aims to accelerate AI research into optimizing high-performance computing systems
When the MIT Lincoln Laboratory Supercomputing Center (LLSC) unveiled its TX-GAIA supercomputer in 2019, it provided the MIT community a powerful new resource for applying artificial intelligence to their research. Anyone at MIT can submit a job to the system, which churns through trillions of operations per second to train models for diverse applications, such as spotting tumors in medical images, discovering new drugs, or modeling climate effects. But with this great power comes the great responsibility of managing and operating it in a sustainable manner—and the team is looking for ways to improve.
“We have these powerful computational tools that let researchers build intricate models to solve problems, but they can essentially be used as black boxes. What gets lost in there is whether we are actually using the hardware as effectively as we can,” says Siddharth Samsi, a research scientist in the LLSC.
To gain insight into this challenge, the LLSC has been collecting detailed data on TX-GAIA usage over the past year. More than a million user jobs later, the team has released the dataset open source to the computing community.
Company’s 3D microchip gives mechanistic insights into human brain
Diseases such as Alzheimer’s and epilepsy will be easier to detect.
A 3D microchip made by a Swiss company will allow scientists to study the complexity of 3D cellular networks. This 3D chip will help to observe complex structures such as the human brain, according to a report published by Labiotech.eu.
Understanding how organs form and how their cells behave is essential to finding the causes and treatment for developmental disorders, as well as understanding certain diseases, said 3Brain.
A microchip that allows scientists to study the complexity of 3D cellular networks at unrivaled scale and precision has been added to 3Brain AG’s brain-on-chip portfolio.
In collaboration with Swiss precision manufacturing experts, CSEM, 3Brain AG made the announcement today (August 22).
The cell-electronic interface technology will also allow scientists to gain novel mechanistic insights into the inner workings of the most complex structure in the universe, the human brain.

E-Project
“These results will have future implications in forensic medicine and genetic diagnosis.”
In 1999, François Brunelle, a Canadian artist, and photographer, began documenting look-alikes in a picture series “I’m not a look-alike!”
The project, undoubtedly, was a massive hit on social media and other parts of the internet, but it also drew the attention of scientists who study genetic relationships.