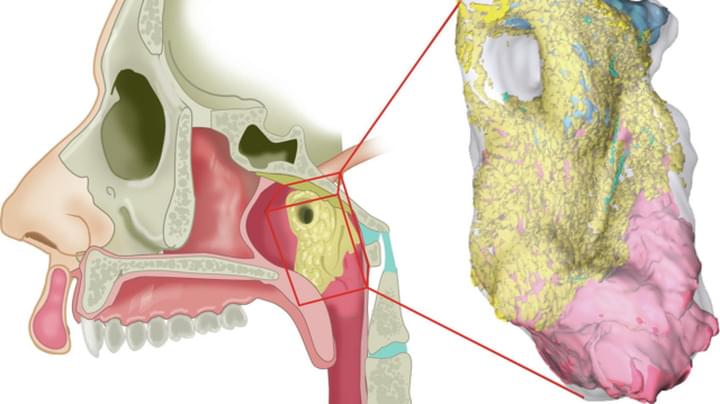
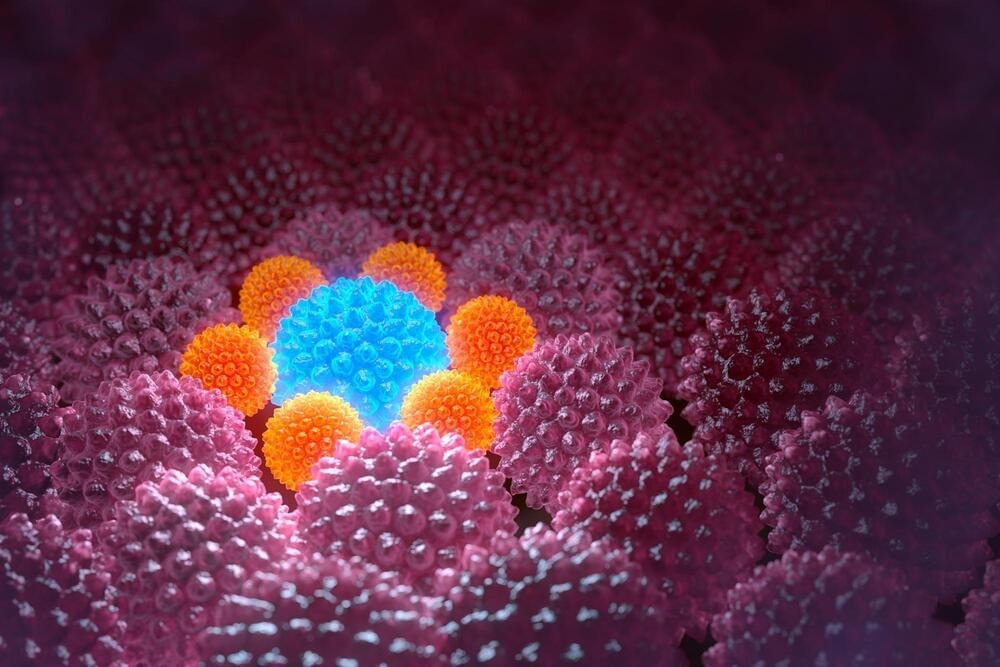
Scientists at the University of California, San Francisco (UCSF) have engineered molecules that act like “cellular glue,” allowing them to direct in precise fashion how cells bond with each other. The discovery represents a major step toward building tissues and organs, a long-sought goal of regenerative medicine.
Adhesive molecules are found naturally throughout the body, holding its tens of trillions of cells together in highly organized patterns. They form structures, create neuronal circuits, and guide immune cells to their targets. Adhesion also facilitates communication between cells to keep the body functioning as a self-regulating whole.
In a new study, published in the December 12, 2022, issue of Nature, researchers engineered cells containing customized adhesion molecules that bound with specific partner cells in predictable ways to form complex multicellular ensembles.