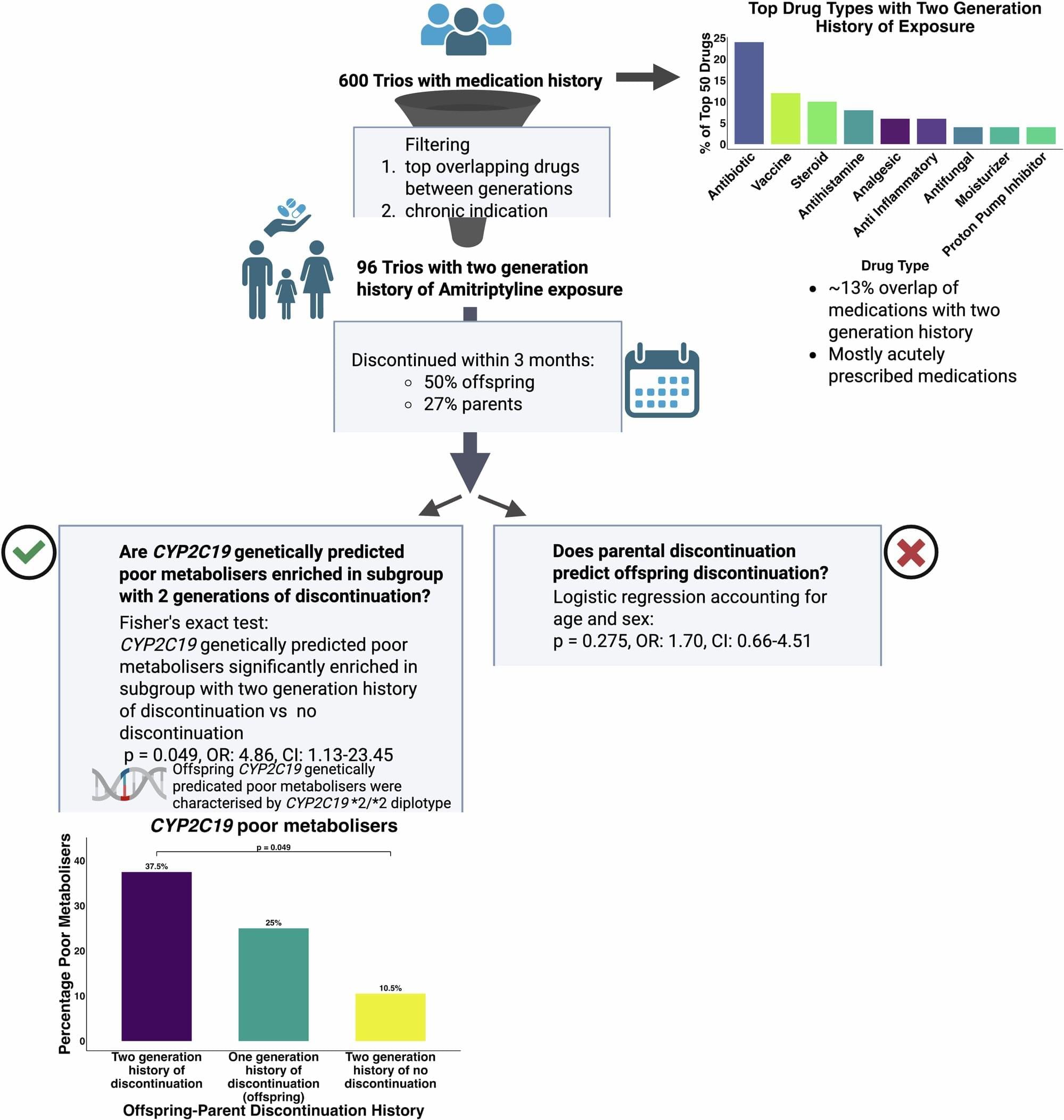
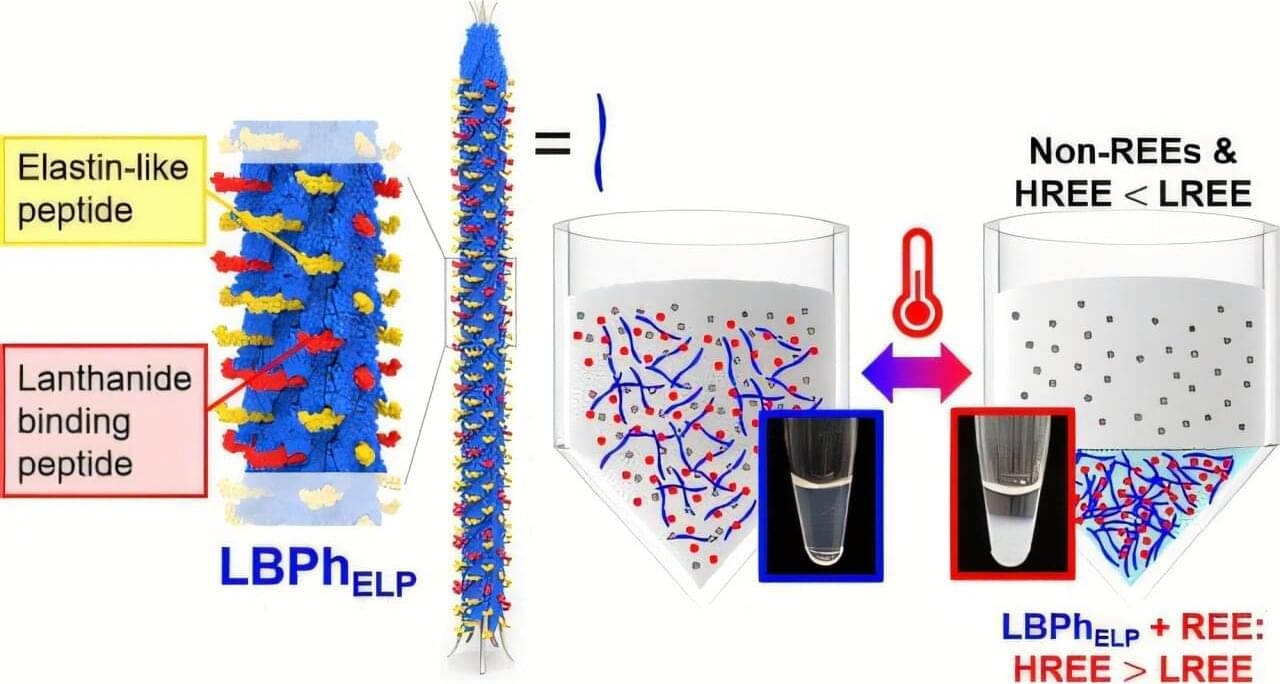
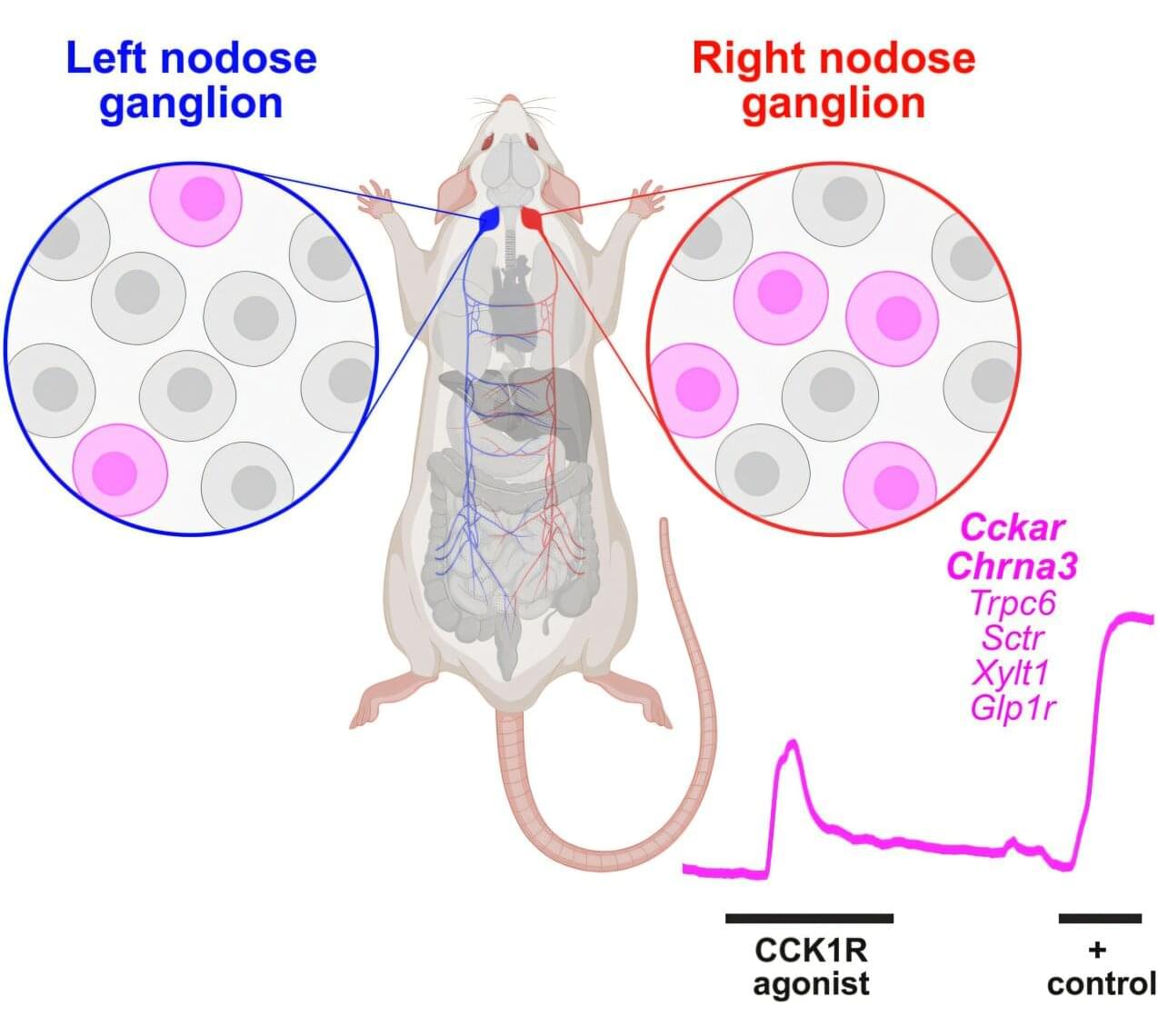
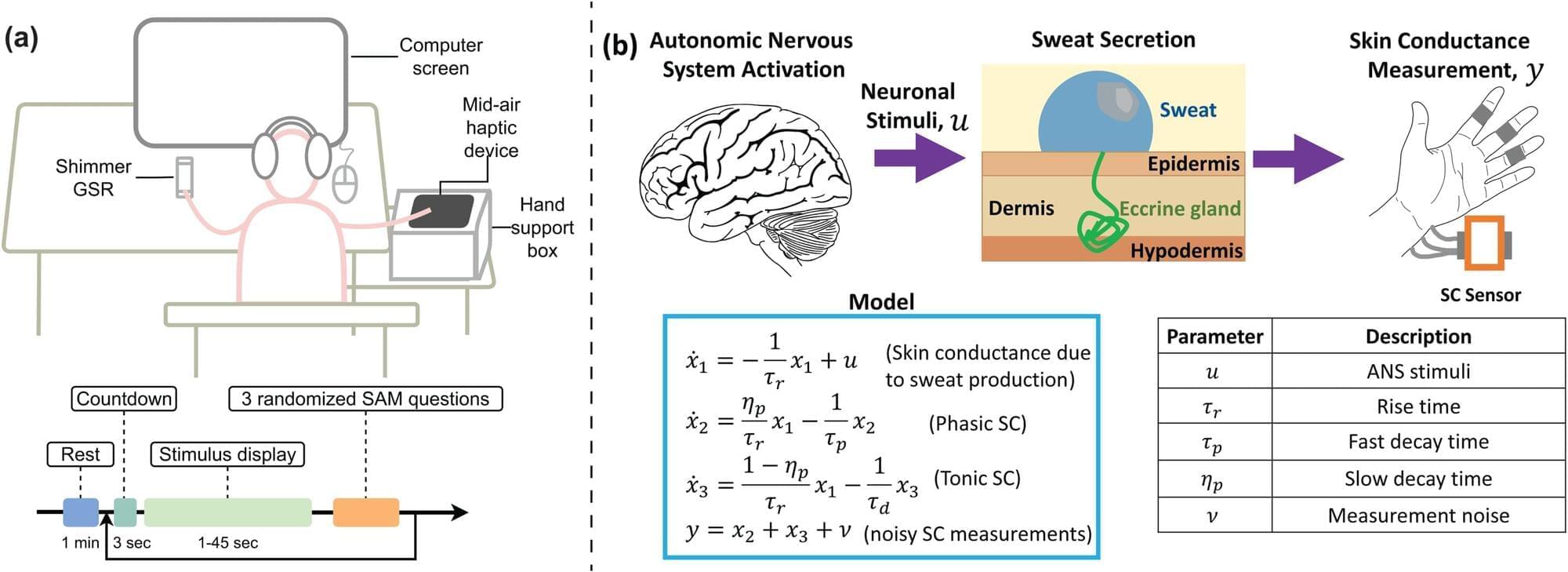
This Neurology Education Teaching Neurovisual by Sutherland and Gummerson details the Head-Impulse-Nystagmus-Test-of-Skew (HINTS) exam, which uses special maneuvers to identify central etiologies of acute vestibular syndrome with greater sensitivity than hyperacute MRI.
Letters to the Editor.
The Letters section represents an opportunity for ongoing author debate and post-publication peer review. View our submission guidelines for Letters to the Editor before submitting your comment.