A groundbreaking study has shown that ALS may actually be an autoimmune disease.


For the first time, a research team from the Senckenberg Center for Human Evolution and Paleoenvironment at the University of Tübingen and the Schöningen Research Center have reconstructed the genomes of an extinct horse species, Equus mosbachensis, from the archaeological site of Schöningen in Lower Saxony, which is approximately 300,000 years old.
Thanks to exceptionally favorable preservation conditions, the researchers were able to identify the oldest DNA yet discovered from an open-air site. Their analyses show that the Schöningen horses belong to a lineage that is considered to be the origin of all modern horses. The study is published in the journal Nature Ecology & Evolution.
Domestic and wild horses, donkeys and zebras all belong to the sole genus of the family Equidae still in existence today. But a look into the fossil record shows that more than 35 different genera and hundreds of now extinct equine species occurred throughout the past.

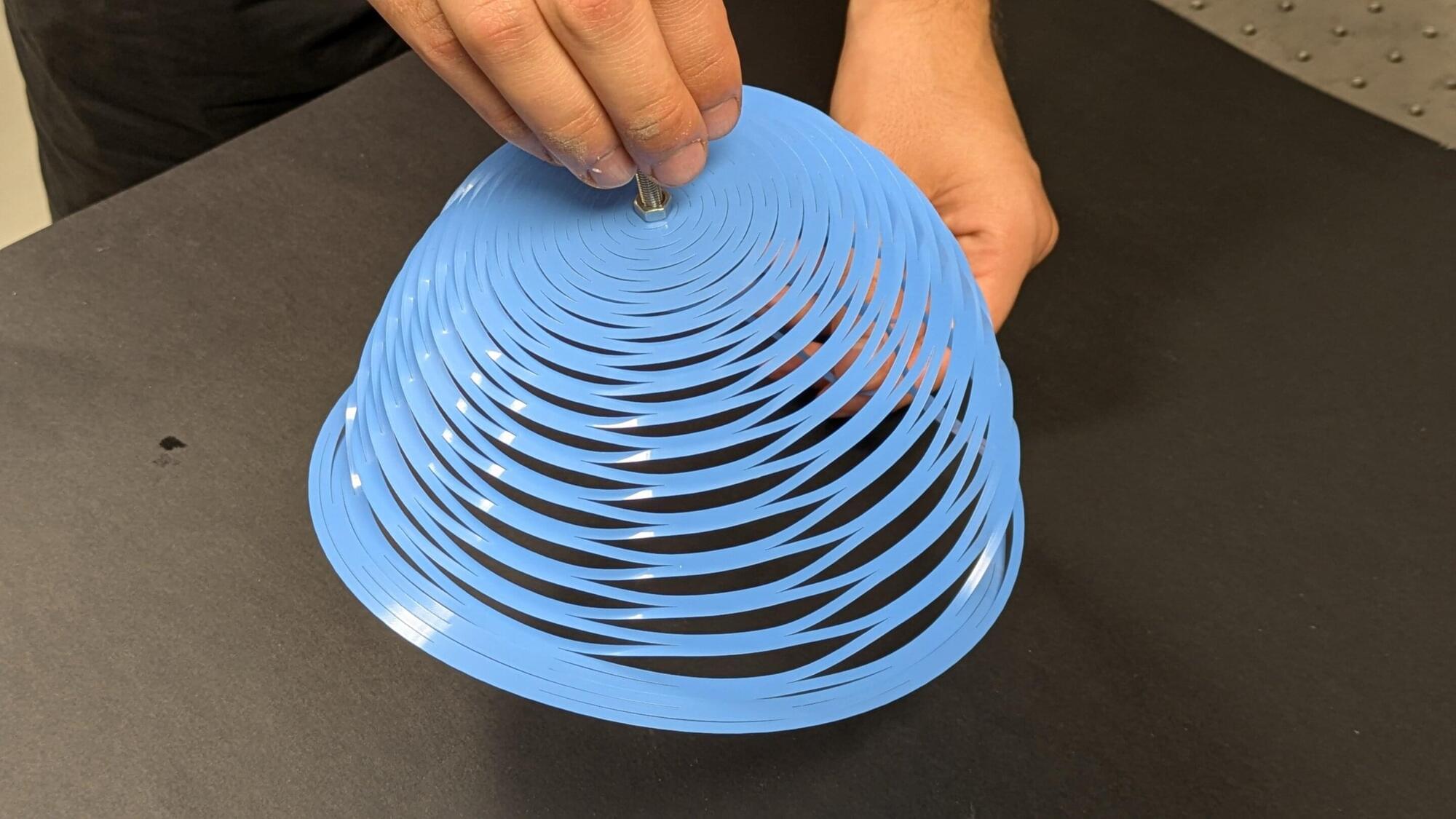
A team of engineers from Polytechnique Montréal report a new and unique parachute concept inspired by the Japanese art of kirigami today in Nature. This simple, robust and low-cost approach has a wide variety of potential applications ranging from humanitarian aid to space exploration.
Kirigami is a technique that modifies the mechanical properties of a sheet of material by making precise folds and cuts to it. Children use it to make snowflakes out of paper, and engineers have used it to create extensible structures, flexible medical devices and deployable spatial structures. However, kirigami techniques have never been applied to parachute production.
The Polytechnique Montréal research team has now changed all that.

Long-term exposure to the industrial solvent trichloroethylene (TCE) outdoors may be linked to an increased risk of Parkinson’s disease, according to a large nationwide study published in Neurology.
TCE is a chemical used in metal degreasing, dry cleaning and other industrial applications. Although TCE has been banned for certain uses, it remains in use today as an industrial solvent and is a persistent environmental pollutant in air, water and soil across the United States. The study does not prove that TCE exposure causes Parkinson’s disease, it only shows an association.
“In this nationwide study of older adults, long-term exposure to trichloroethylene in outdoor air was associated with a small but measurable increase in Parkinson’s risk,” said study author Brittany Krzyzanowski, Ph.D., of Barrow Neurological Institute in Phoenix. “These findings add to a growing body of evidence that environmental exposures may contribute to Parkinson’s disease.”
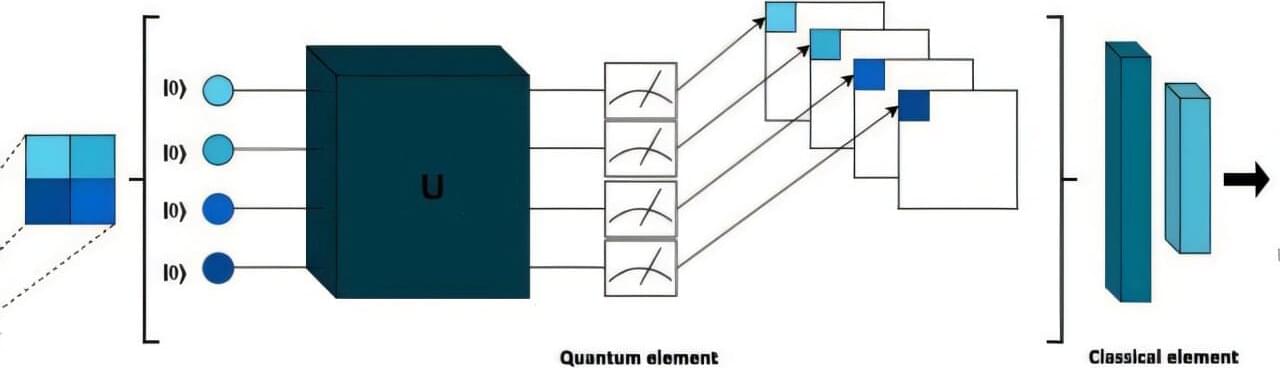
Quantum computing is still in its early stages of development, but researchers have extensively explored its potential uses. A recent study conducted at São Paulo State University (UNESP) in Brazil proposed a hybrid quantum-classical model to support breast cancer diagnosis from medical images.
The work was published as part of the 2025 IEEE 38th International Symposium on Computer-Based Medical Systems (CBMS), organized by the Institute of Electrical and Electronics Engineers (IEEE). In the publication, the authors describe a hybrid neural network that combines quantum and classical layers using an approach known as a quanvolutional neural network (QNN). They applied the model to mammography and ultrasound images to classify lesions as benign or malignant.
“What we wanted to bring to this work was a very basic architecture that used quantum computing but contained a minimum of quantum and classical devices,” says Yasmin Rodrigues, the first author of the study. The work is part of her scientific initiation project, supervised by João Paulo Papa, full professor in the Department of Computing at the Bauru campus of UNESP. Papa also co-authored the article.
Though it might seem like science fiction, scientists are working to build nanoscale molecular machines that can be designed for myriad applications, such as “smart” medicines and materials. But like all machines, these tiny devices need a source of power, the way electronic appliances use electricity or living cells use ATP (adenosine triphosphate, the universal biological energy source).
Researchers in the laboratory of Lulu Qian, Caltech professor of bioengineering, are developing nanoscale machines made out of synthetic DNA, taking advantage of DNA’s unique chemical bonding properties to build circuits that can process signals much like miniature computers. Operating at billionth-of-a-meter scales, these molecular machines can be designed to form DNA robots that sort cargos or to function like a neural network that can learn to recognize handwritten numerical digits.
One major challenge, however, has remained: how to design and power them for multiple uses.
Imagine watching a favorite movie when suddenly the sound stops. The data representing the audio is missing. All that’s left are images. What if artificial intelligence (AI) could analyze each frame of the video and provide the audio automatically based on the pictures, reading lips and noting each time a foot hits the ground?
That’s the general concept behind a new AI that fills in missing data about plasma, the fuel of fusion, according to Azarakhsh Jalalvand of Princeton University. Jalalvand is the lead author on a paper about the AI, known as Diag2Diag, that was recently published in Nature Communications.
“We have found a way to take the data from a bunch of sensors in a system and generate a synthetic version of the data for a different kind of sensor in that system,” he said. The synthetic data aligns with real-world data and is more detailed than what an actual sensor could provide. This could increase the robustness of control while reducing the complexity and cost of future fusion systems. “Diag2Diag could also have applications in other systems such as spacecraft and robotic surgery by enhancing detail and recovering data from failing or degraded sensors, ensuring reliability in critical environments.”