Minimizing the use of sedatives during operations could shorten hospital stays and reduce the risk of complications.


A susceptibility to gain weight may be written into molecular processes of human cells, a Washington State University study indicates.
The proof-of-concept study with a set of 22 twins found an epigenetic signature in buccal or cheek cells appearing only for the twins who were obese compared to their thinner siblings. With more research, the findings could lead to a simple cheek swab test for an obesity biomarker and enable earlier prevention methods for a condition that effects 50% of U.S. adults, the researchers said.
“Obesity appears to be more complex than simple consumption of food. Our work indicates there’s a susceptibility for this disease and molecular markers that are changing for it,” said Michael Skinner, a WSU professor of biology and corresponding author of the study published in the journal Epigenetics.

The device shows promise in a 62-year-old advanced-stage Parkinson’s patient.
In a major medical development, a man battling Parkinson’s disease has defied the odds and regained the ability to walk, thanks to a pioneering implanted device in his spinal cord.
The remarkable breakthrough promises new hope for countless individuals worldwide who have been affected by Parkinson’s disease and its associated debilitating walking difficulties.
An enzyme that may help some breast cancers spread can be stopped with an antibody created in the lab of Cold Spring Harbor Laboratory Professor Nicholas Tonks. With further development, the antibody might offer an effective drug treatment for those same breast cancers.
The new antibody targets an enzyme called PTPRD that is overabundant in some breast cancers. PTPRD belongs to a family of molecules known as protein tyrosine phosphatases (PTPs), which help regulate many cellular processes. They do this by working in concert with enzymes called kinases to control how other proteins inside cells behave. Kinases add small chemical regulators called phosphates to proteins. PTPs take them off.
Disruptions in the addition or removal of phosphates can contribute to inflammation, diabetes, and cancer. Some disruptions can be corrected with kinase-blocking drugs.

Researchers led by Mroj Alassaf at the Fred Hutchinson Cancer Research Center in the United States have discovered a link between obesity and neurodegenerative disorders like Alzheimer’s disease.
Using the common fruit fly, the research shows that a high-sugar diet—a hallmark of obesity—causes insulin resistance in the brain, which in turn reduces the ability to remove neuronal debris, thus increasing the risk of neurodegeneration.
Publishing November 7 in the open access journal PLOS Biology, the research will impact therapies designed to reduce the risk of developing neurodegenerative diseases.

Viktor Frankl’s school of psychology based around finding the meaning of your life: https://www.freethink.com/health/viktor-frankl
Not having a meaningful life can be dreadful, and one psychologist thought it was the root of many neuroses. His ideas became Logotherapy.
As heart disease continues to be the leading cause of death in America, experts say these small lifestyle changes can help keep your heart at its healthiest.
“When the data is fulsome and accurate and has a large enough sample size, AI will be able to identify patterns and correlations that humans might struggle to see, especially when they require two or more factors or have seemingly contrarian conclusions,” Phil Siegel, the founder of the Center for Advanced Preparedness and Threat Response Simulation, told Fox News Digital.
Cancer is a malignant disease referring to the uncontrollable proliferation of mutated cells. Millions of individuals are affected by cancer each year in the United States alone. Unfortunately, treatment is limited due to the heterogeneity of the disease and different components, which drive the disease to progress. The proliferation of cells can occur anywhere in the body including different organs such as breast, lung, pancreas, and head and neck. Cancer can also affect the reproductive tract in both men and women including the testes and ovaries, respectively. Particularly, ovarian cancer is linked with breast cancer and can result in infertility due to late detection. Due to limited therapeutic efficacy in ovarian cancer, more research is necessary for a meaningful solution. Different groups are working to more effectively target ovarian cancer through different biologic approaches.
Dr. David B. Weiner and his team from the Wistar Institute recently published an article in Science Advances demonstrating enhanced immunotherapeutic effects in ovarian cancer patients. Immunotherapy refers to a form of cancer therapy that directs the immune system to attack the tumor. In many immunotherapies, immune cells, such as T cells, are activated to kill tumors. This is a unique approach to target cancer compared to chemotherapy or radiation, which tries to directly kill tumor cells and elicit an immune response. Immunotherapy allows the immune system to recognize the tumor and react through the body’s immune system. One prominent immune cell includes natural killer (NK) cells which responsible for initial lying or killing of foreign particles. Novel work has tried to engineer NK cells to target tumors by recognizing unique receptors on its surface.
Weiner’s team and collaborator, Mohamed Abdel-Mohsen, have engineered monoclonal antibodies to engage NK cells to lyse cancer. Interestingly, the team demonstrated this immunotherapeutic regimen optimized preclinical output in mice when combined with checkpoint inhibitors, another type of immunotherapy. The group engineered antibodies to target a glyco-immune marker on most NK cells referred to as Sialic acid-binding immunoglobulin-type lectin (Siglec-7). The novel combination strategy targets NK cells through Siglec-7 and T cells to optimize immune response against tumor cells. The monoclonal antibody (mAb) targeting Siglec-7 allows NK cells to become activated and kill ovarian cancer cells without killing non-cancer cells, which improve specificity and reduce toxicity for patients. Consequently, this antibody resulted in generating a new class of NK cell engagers (NKCE).
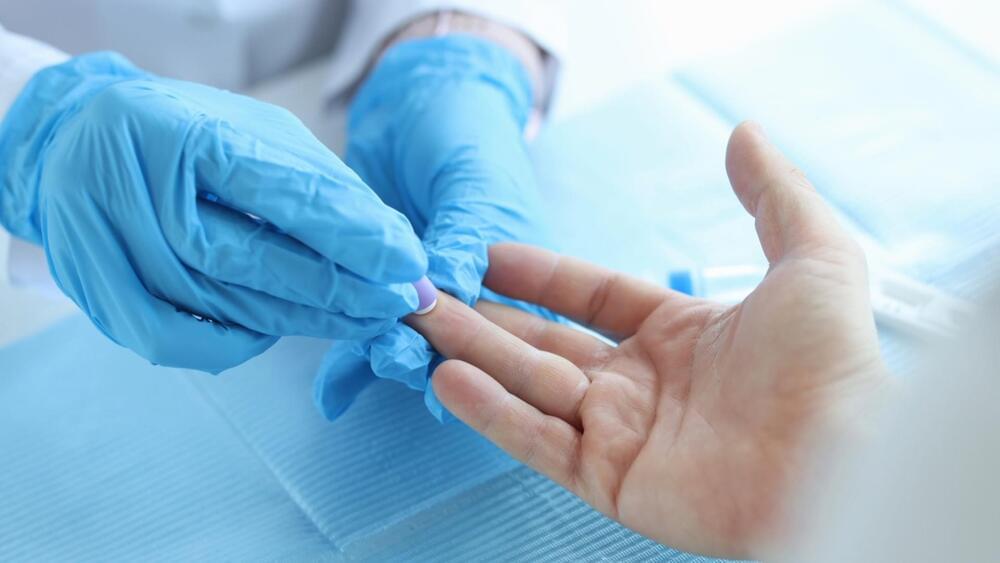
Link :- https://interestingengineering.com/science/finger-prick-test…tent=Nov07
Megaflopp/iStock.
The significance of such a test lies in its non-invasive nature and the potential to detect brain tumors at an earlier stage, which can be critical for timely treatment and improved patient outcomes.

Germany: A recent study published in the International Forum of Allergy & Rhinology has shown the effectiveness of the probiotic treatment with Enterococcus faecalis in reducing the symptoms of allergic rhinitis after four weeks.
The results are in line with similar studies showing the beneficial effects of E. faecalis. Furthermore, the data showed that allergic symptoms were alleviated during the COVID-19 pandemic situation. This is in line with previous studies and might be explained by a reduced confrontation with pollen due to masks, psychological factors, or lockdown situations.
Although allergic rhinitis treatment has been proven to be effective, it is expensive, does not completely resolve symptoms, and is related to side effects. Previous studies have indicated that probiotics may be a new promising treatment for allergic rhinitis. Michael Schaefer, Medical School Berlin, Berlin, Germany, and colleagues aimed to evaluate the effects of a single bacterial strain, Enterococcus faecalis on symptoms of allergic rhinitis. Beneficial effects of E. faecalis have been reported before but not in seasonal allergic rhinitis.