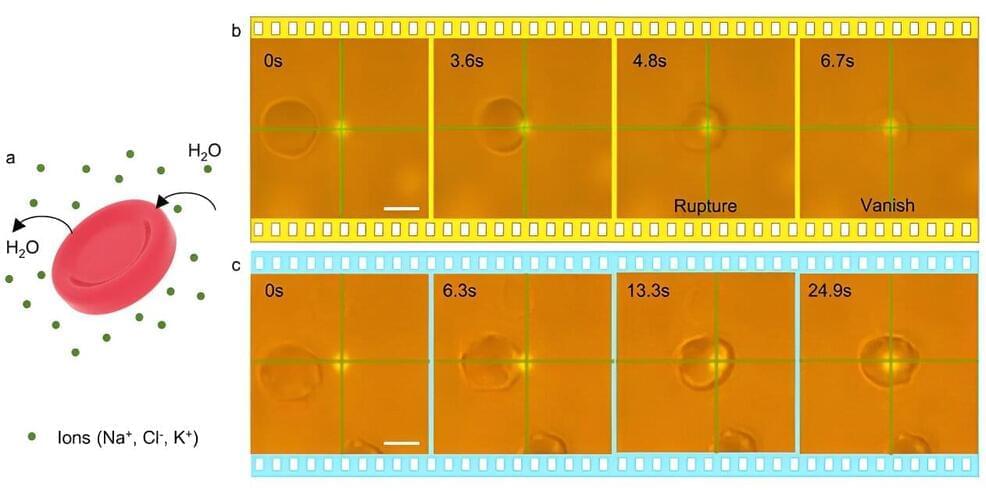
This post is also available in:  עברית (Hebrew)
עברית (Hebrew)
A revolutionary brain implant invented by a team of neuroscientists, neurosurgeons, and engineers can transform thoughts into speech. This technology will hopefully help people who cannot speak because of neurological conditions be able to communicate once more.
Gregory Cogan, a professor of neurology at Duke University’s School of Medicine and one of the lead researchers in the project, explains: “There are many patients who suffer from debilitating motor disorders, like Amyotrophic Lateral Sclerosis (ALS) or locked-in syndrome, that can impair their ability to speak… But the current tools available to allow them to communicate are generally very slow and cumbersome.”