The model can help evolve “better methods for growing cells for blood transfusions, novel cell therapies, and hematopoietic stem cell transplants.”
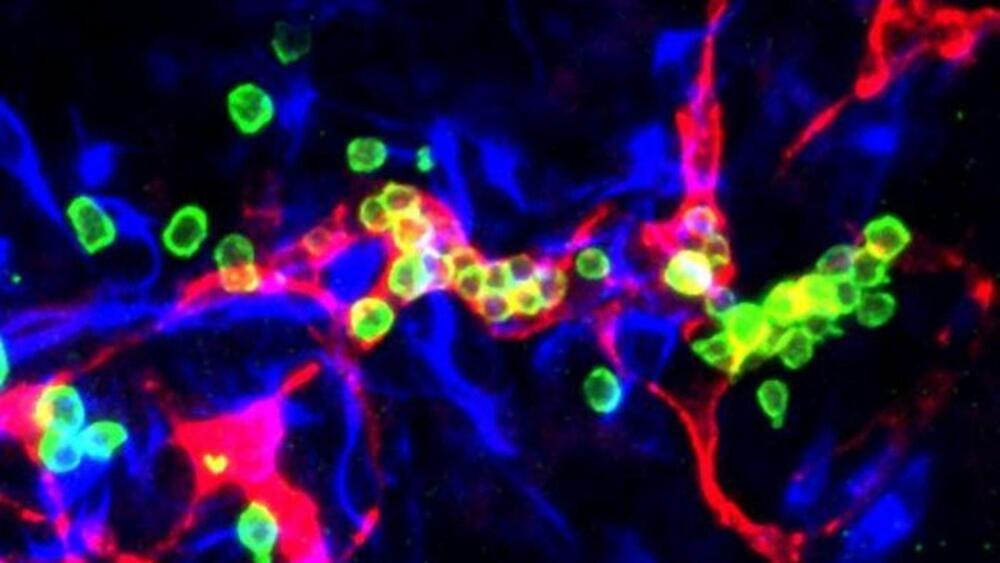
Remarkably, heX-Embryoid models developed structures akin to blood islands, the initial sites supporting the generation of blood cells in developing embryos. The study identified progenitors for red blood cells, platelets, and various white blood cell types—a pivotal advancement in the field, according to the team.
Researchers claim the model successfully replicated a process closely resembling the initial stages of blood production in humans. “This is exciting because there are extensive possibilities to apply this model to better understand how blood is formed and develop better methods for growing cells for blood transfusions, novel cell therapies, and hematopoietic stem cell transplants,” said Mo Ebrahimkhani, senior author and an associate professor at the Pittsburgh Liver Institute and the Department of Bioengineering at Pitt, in a statement.
Versatile characteristics
HeX-Embryoids exhibit distinctive characteristics, such as the absence of the trophoblast layer responsible for placenta formation and an open yolk sac, lacking a closed cavity. These limitations preclude the embryoids from attaining the status of a genuine embryo or possessing the potential for complete developmental implantation.