In Episode 3 of the Lifespan Podcast, Dr. David Sinclair and Matthew LaPlante dive deeply into the science of non-dietary interventions that mimic adversity and promote health. They begin by highlighting how different types of physical activity (i.e., low-intensity aerobic exercise, high-intensity aerobic exercise, and weight training) protect against age-related disease and enhance longevity. David and Matthew additionally highlight the latest evidence behind hyperbaric oxygen therapy, cold therapy, and heat therapy. As they discuss different adversity mimetics, they also explain how these interventions influence aging at the molecular and physiological levels.
Category: biotech/medical – Page 1,044
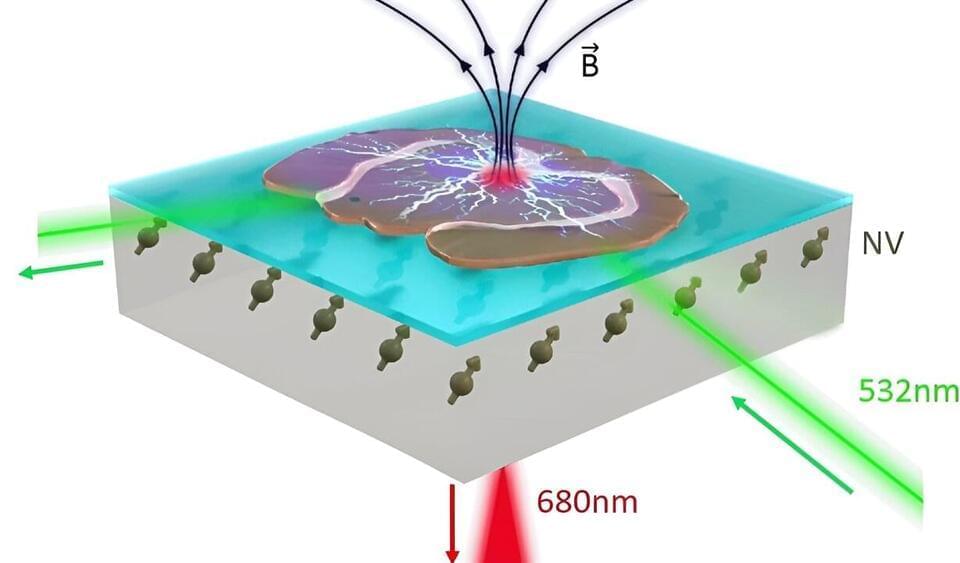
Diamond quantum sensors measure neuron activity
A recent study by European scientists shows that highly sensitive sensors based on color centers in a diamond can be used to record electrical activity from neurons in living brain tissue. The work is published in the journal Scientific Reports.
Before people encounter symptoms of brain diseases such as dementia, slight changes have usually occurred already in the brain tissue. It may be that parts of the brain are swelling up or clumps of proteins are forming. These small changes might influence how nerve cells in the brain signal each other and communicate, how information is processed and memorized.
Medical scientists want to study these minor changes that occur in the very early stages of a disease. That way, the intention is to learn more about the causes of the disease to provide new insights and more efficient treatments. Today, microscopic studies on the brain are performed with one of two strategies: Optical inspection of brain tissue samples from animals or deceased patients that suffer from the studied disease or measurements of the signals from the nerve cells using wires, coloring, or light.
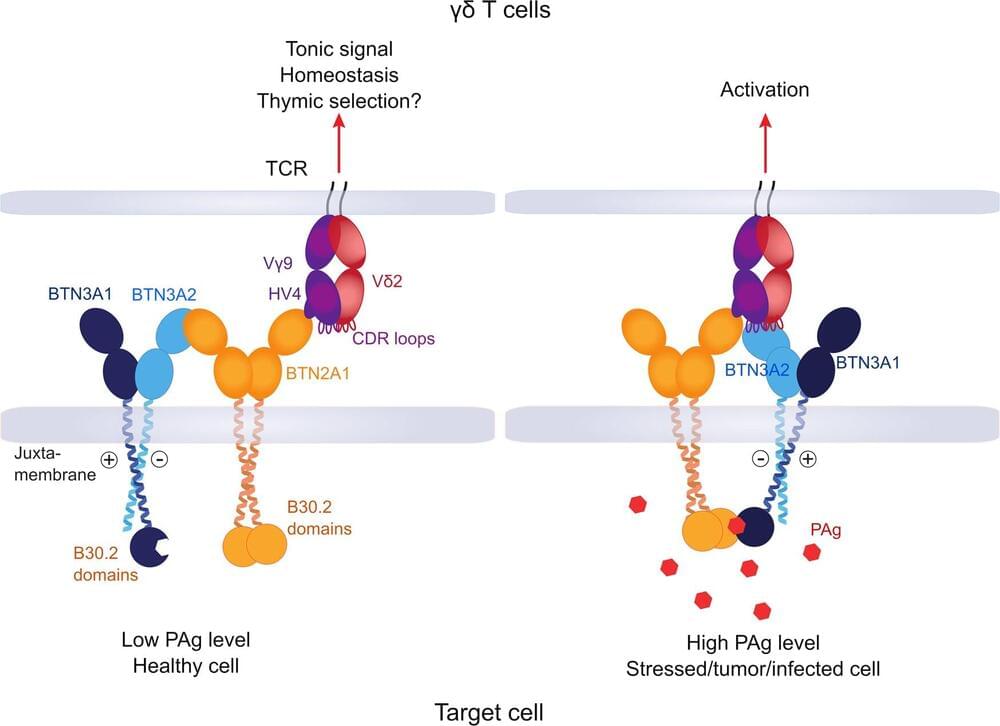
Study offers new insights into how immune cells recognize their enemies
In order for immune cells to do their job, they need to know against whom they should direct their attack. Research teams at the University of Würzburg have identified new details in this process.
As complicated as their name is, they are important for the human organism in the fight against pathogens and cancer: Vγ9Vδ2 T cells are part of the immune system and, as a subgroup of white blood cells, fight tumor cells and cells infected with pathogens. They recognize their potential victims by their altered cell metabolism.
Research teams from the University of Würzburg and the University Hospital of Würzburg, together with groups in Hamburg, Freiburg, Great Britain and the U.S., have now gained new insights into how these cells manage to look inside the cell. Thomas Herrmann, Professor of Immunogenetics at the Institute of Virology and Immunobiology and his colleague Dr. Mohindar Karunakaran at Julius-Maximilians-Universität Würzburg (JMU), were responsible for the study published in the journal Nature Communications.

Researchers crack the cellular code on protein folding, offering hope for many new therapeutic avenues
While we often think of diseases as caused by foreign bodies—bacteria or viruses—there are hundreds of diseases affecting humans that result from errors in cellular production of proteins.
A team of researchers led by the University of Massachusetts Amherst leveraged the power of cutting-edge technology, including an innovative technique called glycoproteomics, to unlock the carbohydrate-based code that governs how certain classes of proteins form themselves into the complex shapes necessary to keep us healthy.
The research, published in the journal Molecular Cell, explores members of a family of proteins called serpins, which are implicated in a number of diseases. The research is the first to investigate how the location and composition of carbohydrates attached to the serpins ensure that they fold correctly.
DNA-folding nanorobots can manufacture limitless copies of themselves
Researchers have demonstrated a programmable nano-scale robot, made from a few strands of DNA, that’s capable of grabbing other snippets of DNA, and positioning them together to manufacture new UV-welded nano-machines – including copies of itself.
The robots, according to New Scientist, are created using just four strands of DNA, and measure just 100 nanometers across, so about a thousand of them could squeeze up into a line the width of a human hair.
The team, from New York University, the Ningbo Cixi Institute of Biomechanical Engineering, and The Chinese Academy of Sciences, says the robots surpass previous efforts, which were only able to assemble pieces into two-dimensional shapes. The new bots are able to use “multiple-axis precise folding and positioning” to “access the third dimension and more degrees of freedom.”
Unlocking the Secrets of Aging: Science, Studies, and Interventions for Longer, Healthier Lives
In this episode, Dr. David Sinclair and co-host Matthew LaPlante discuss why we age. In doing so, they discuss organisms that have extreme longevity, the genes that control aging (mTOR, AMPK, Sirtuins), the role of sirtuin proteins as epigenetic regulators of aging, the process of “ex-differentiation” in which cells begin to lose their identity, and how all of this makes up the “Information Theory of Aging”, and the difference between “biological age” and “chronological age” and how we can measure biological age through DNA methylation clocks. #Aging #DavidSinclair #Longevity
NMN, NR, Resveratrol, Metformin & Other Longevity Molecules
In this week’s episode, Dr. David Sinclair and co-host Matthew LaPlante zero in on drugs and supplements that have been reported to combat aspects of aging. They share the latest experimental and clinical data for NAD boosters (these being NR, NMN, NAD IV drips and shots), resveratrol, fisetin, quercetin, rapamycin, spermidine, metformin, and berberine.
Brain implants revive cognitive abilities long after traumatic brain injury
The results of the clinical trial were published Dec. 4 in Nature Medicine.
More than 5 million Americans live with the lasting effects of moderate to severe traumatic brain injury — difficulty focusing, remembering and making decisions. Though many recover enough to live independently, their impairments prevent them from returning to school or work and from resuming their social lives.

Brain Area Associated With Impulse Control Discovered
Summary: A new study identified the right inferior frontal gyrus (rIFG) as a central regulator in the brain’s inhibitory control circuit.
Using dynamic causal modeling and fMRI on a sample of 250 participants, the study reveals that the rIFG significantly influences the caudate nucleus and thalamus during response inhibition tasks. This research also shows gender differences in brain function: women have distinct neural patterns in the thalamus, and overall, better inhibitory control correlates with stronger neural communication from the thalamus to the rIFG.
These findings provide valuable insights for developing neuromodulation therapies for mental and neurological disorders with inhibitory control deficits.