Awarded the 2023 Nobel Prize in Chemistry, quantum dots have a wide variety of applications ranging from displays and LED lights to chemical reaction catalysis and bioimaging. These semiconductor nanocrystals are so small – on the order of nanometers – that their properties, such as color, are size dependent, and they start to exhibit quantum properties. This technology has been really well developed, but only in the visible spectrum, leaving untapped opportunities for technologies in both the ultraviolet and infrared regions of the electromagnetic spectrum.
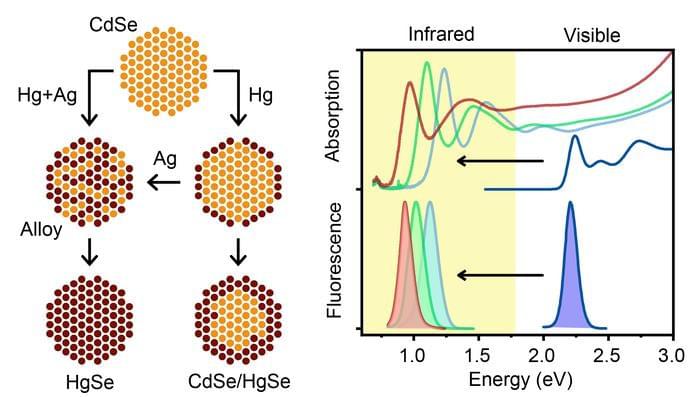
In new research published in Nature Synthesis (“Interdiffusion-enhanced cation exchange for HgSe and HgCdSe nanocrystals with infrared bandgaps”), University of Illinois at Urbana-Champaign bioengineering professor Andrew Smith and postdoctoral researcher Wonseok Lee have developed mercury selenide (HgSe) and mercury cadmium selenide (HgCdSe) nanocrystals that absorb and emit in the infrared, made from already well-developed, visible spectrum cadmium selenide (CdSe) precursors. The new nanocrystal products retained the desired properties of the parent CdSe nanocrystals, including size, shape and uniformity.
“This is the first example of infrared quantum dots that are at the same level of quality as the ones that are in the visible spectrum,” Smith says.