Sources and further reading:
- Bridge RNAs direct programmable recombination of target and donor DNA https://www.nature.com/articles/s4158…
- Structural mechanism of bridge RNA-guided recombination https://www.nature.com/articles/s4158…
Sources and further reading:
- Bridge RNAs direct programmable recombination of target and donor DNA https://www.nature.com/articles/s4158…
- Structural mechanism of bridge RNA-guided recombination https://www.nature.com/articles/s4158…

Chinese scientists have developed a method using genetic engineering to potentially enhance brain-computer interface (BCI) technology by enlarging neurons for better signal transmission.
The researchers, with the Chinese Academy of Sciences’ National Centre for Nanoscience…
Gene sequence could be implanted with electrodes to make neurons larger and easier to ‘read’ in quest for better mind control of devices.
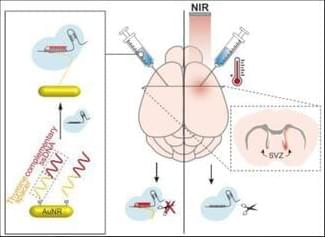
A nanoparticle formulation, using oligonucleotide chemistry, able to release a gene editing system with single cell resolution after near infrared laser activation. The full potential of the formulation was demonstrated in the brain after intracerebral and intranasal administrations. The spot of the laser defined the region of gene editing.
Noninvasive braincomputer interfaces could vastly improve brain computer control.
Over the past two decades, the international biomedical research community has demonstrated increasingly sophisticated ways to allow a person’s brain to communicate with a device, allowing breakthroughs aimed at improving quality of life, such as access to computers and the internet, and more recently control of a prosthetic limb. DARPA has been at the forefront of this research.
The state of the art in brain-system communications has employed invasive techniques that allow precise, high-quality connections to specific neurons or groups of neurons. These techniques have helped patients with brain injury and other illnesses. However, these techniques are not appropriate for able-bodied people. DARPA now seeks to achieve high levels of brain-system communications without surgery, in its new program, Next-Generation Nonsurgical Neurotechnology (N3).
“DARPA created N3 to pursue a path to a safe, portable neural interface system capable of reading from and writing to multiple points in the brain at once,” said Dr. Al Emondi, program manager in DARPA’s Biological Technologies Office (BTO). “High-resolution, nonsurgical neurotechnology has been elusive, but thanks to recent advances in biomedical engineering, neuroscience, synthetic biology, and nanotechnology, we now believe the goal is attainable.”
There are different types of biotechnology protocols for genome/gene editing (GE), but the preferred one is the Clustered Regularly Interspaced Short Palynodromic Repeat (CRISPR) Cas9 system. Advantages include precision, the ability to design variants tailored to needs, and optimal operational cost and time.

University of Arizona researchers have developed an ‘attomicroscopy’ technique using a novel ultrafast electron microscope that captures moving electrons in unprecedented detail, paving the way for significant scientific breakthroughs in physics and other fields.
Imagine having a camera so advanced that it can capture freeze-frame images of a moving electron—an object so fast it could orbit the Earth multiple times in just a second. Researchers at the University of Arizona have developed the world’s fastest electron microscope capable of this remarkable feat.
They believe their work will lead to groundbreaking advancements in physics, chemistry, bioengineering, materials sciences, and more.

The future of medicine lies in synthetic biology! In this video, you’ll learn how synthetic biology is used in healthcare and why it can help develop cancer treatments and much more.
Are you interested in furthering the field of biology or medicine? Visit uvu.edu to carve your path.
Instagram: / utah.valley.university.
Facebook: / utahvalleyuniversity.
LinkedIn: / utah-valley-university.
Twitter: / uvu.