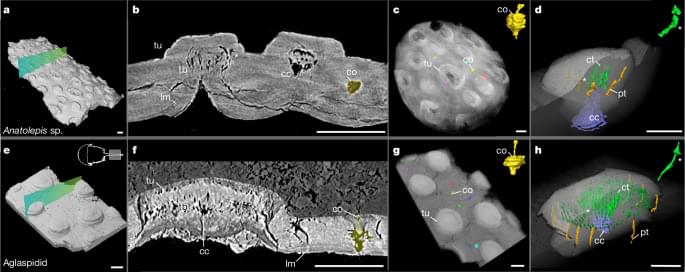
This study critically distinguishes between AI Agents and Agentic AI, offering a structured conceptual taxonomy, application mapping, and challenge analysis to clarify their divergent design philosophies and capabilities. We begin by outlining the search strategy and foundational definitions, characterizing AI Agents as modular systems driven by Large Language Models (LLMs) and Large Image Models (LIMs) for narrow, task-specific automation. Generative AI is positioned as a precursor, with AI Agents advancing through tool integration, prompt engineering, and reasoning enhancements. In contrast, Agentic AI systems represent a paradigmatic shift marked by multi-agent collaboration, dynamic task decomposition, persistent memory, and orchestrated autonomy. Through a sequential evaluation of architectural evolution, operational mechanisms, interaction styles, and autonomy levels, we present a comparative analysis across both paradigms. Application domains such as customer support, scheduling, and data summarization are contrasted with Agentic AI deployments in research automation, robotic coordination, and medical decision support. We further examine unique challenges in each paradigm including hallucination, brittleness, emergent behavior, and coordination failure and propose targeted solutions such as ReAct loops, RAG, orchestration layers, and causal modeling. This work aims to provide a definitive roadmap for developing robust, scalable, and explainable AI agent and Agentic AI-driven systems. >AI Agents, Agent-driven, Vision-Language-Models, Agentic AI Decision Support System, Agentic-AI Applications