As Venezuela’s economy continues to endure hyperinflation, more businesses start accepting crypto.



Stem Cell Neurotherapy, which has achieved successful results with Parkinson’s, Essential Tremor, and Brain Tumor, can generate new cells and tissues in the lungs, liver, kidney, etc., to replace those cells and tissues that have been infected by COVID-19. This will improve the lung microenvironment, protect lung alveoli epithelial cells, and restore healthy functioning lungs.
These new cells will eliminate the fever, coughing, headaches, breathing problems, and other symptoms related to COVID-19.
At the 9:03 minute mark on the Stem Cell Neurotherapy recording, you will hear the therapeutic message:
“Your stem cells are going to transform themselves into new lung, liver, kidney, and other cells to replace those cells that have been infected by the Coronavirus. This will eliminate the inflammation, fever, breathing difficulties, coughing, and other symptoms caused by the virus.
Your body is going to produce healing hormones, messenger molecules, and proteins which will transform the stem cells into mature, well-functioning connections and tissues. These new cells and tissues are going to replace those cells and tissues that have been infected by the virus.
Your stem cells are going to transform themselves into T-Cells, B-Cells, and Natural Killer Cells, which will seek out, identify, attack, and destroy all the Coronavirus cells in your entire body.”


China plans to send an astronaut to the Moon in about a decade and then build a base there. Its lunar rover on the far side has driven about 450 metres so far.
The next big mission for Beijing is to land a probe on Mars, with liftoff expected this year.
Space agency tests new design that is billed to replace current Shenzhou module, a copy of Russia’s Soyuz.

The U.S. space agency National Aeronautics Space Administration (NASA), European Space Agency (ESA), and Japan Aerospace Exploration Agency (JAXA) are inviting coders, entrepreneurs, scientists, designers, storytellers, makers, builders, artists, and technologists to participate in a virtual hackathon May 30–31 dedicated to putting open data to work in developing solutions to issues related to the COVID-19 pandemic.
During the global Space Apps COVID-19 Challenge, participants from around the world will create virtual teams that – during a 48-hour period – will use Earth observation data to propose solutions to COVID-19-related challenges ranging from studying the coronavirus that causes COVID-19 and its spread to the impact the disease is having on the Earth system. Registration for this challenge opens in mid-May.
“There’s a tremendous need for our collective ingenuity right now,” said Thomas Zurbuchen, associate administrator for NASA’s Science Mission Directorate. “I can’t imagine a more worthy focus than COVID-19 on which to direct the energy and enthusiasm from around the world with the Space Apps Challenge that always generates such amazing solutions.”
The unique capabilities of NASA and its partner space agencies in the areas of science and technology enable them to lend a hand during this global crisis. Since the start of the global outbreak, Earth science specialists from each agency have been exploring ways to use unique Earth observation data to aid understanding of the interplay of the Earth system – on global to local scales – with aspects of the COVID-19 outbreak, including, potentially, our ability to combat it. The hackathon will also examine the human and economic response to the virus.


In 2033, people can be “uploaded” into virtual reality hotels run by 6 tech firms. Cash-strapped Nora lives in Brooklyn and works customer service for the luxurious “Lakeview” digital afterlife. When L.A. party-boy/coder Nathan’s self-driving car crashes, his high-maintenance girlfriend uploads him permanently into Nora’s VR world. Upload is created by Greg Daniels (The Office).
face_with_colon_three could heal body parts in humans.
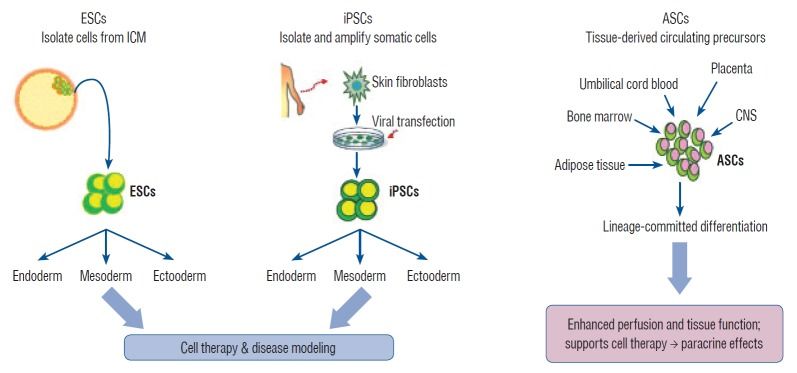
The generation of human induced pluripotent stem cells (iPSCs) from somatic cells using gene transfer opens new areas for precision medicine with personalized cell therapy and encourages the discovery of essential platforms for targeted drug development. iPSCs retain the genome of the donor, may regenerate indefinitely, and undergo differentiation into virtually any cell type of interest using a range of published protocols. There has been enormous interest among researchers regarding the application of iPSC technology to regenerative medicine and human disease modeling, in particular, modeling of neurologic diseases using patient-specific iPSCs. For instance, Parkinson’s disease, Alzheimer’s disease, and spinal cord injuries may be treated with iPSC therapy or replacement tissues obtained from iPSCs. In this review, we discuss the work so far on generation and characterization of iPSCs and focus on recent advances in the use of human iPSCs in clinical setting.
Stem cells exhibit the capacity of self-renewal and may undergo differentiation into various tissue types. These are divided into pluripotent stem cells (PSCs; embryonic stem cells [ESCs] and induced pluripotent stem cells [iPSCs]) and multipotent stem cells (adult stem cells [ASCs]) based on their differentiation capacity [45]. PSCs, including ESCs derived from embryos and iPSCs derived by gene transfer, may undergo indefinite proliferation and differentiate into different types of tissues depending on the treatment conditions [86]. Multipotent stem cells, however, may be obtained from tissue-derived precursors (umbilical cord blood, bone marrow, adipose tissue, placenta, or blood), which are already grown tissues.
Tesla has patented a new battery cell with a tabless electrode that Elon Musk hypes as “way more important than it sounds.”
In the new patent application published today, Tesla explains constraints with current battery cells:
Current cells use a jelly-roll design in which the cathode, anode, and separators are rolled together and have a cathode tab and an anode tab to connect to the positive and negative terminals of the cell can. The path of the current necessarily travels through these tabs to connectors on the outside of the battery cell. However, ohmic resistance is increased with distance when current must travel all the way along the cathode or anode to the tab and out of the cell. Furthermore, because the tabs are additional components, they increase costs and present manufacturing challenges.
