Is up at Cosmoquest.
Some antidepressants could potentially be used to treat a wide range of diseases caused by bacteria living within cells, according to work by researchers in the Virginia Commonwealth University School of Medicine and collaborators at other institutions.
Research published in the April print edition of the journal Life Science Alliance, shows that antidepressant drugs called FIASMAs, including desipramine, amitriptyline, and nortriptyline, halt the growth or kill four different intracellular bacterial pathogens in tissue cell culture and animal models.
“Antibiotic options for diseases caused by intracellular bacteria are limited because many of these drugs cannot penetrate our cell membranes. In essence, the bacteria are protected,” said Jason Carlyon, Ph.D., leader of the study and professor in the VCU Department of Microbiology and Immunology.
The cells of most patients’ cancers are resistant to a class of drugs, called proteasome inhibitors, that should kill them. When studied in the lab, these drugs are highly effective, yet hundreds of clinical trials testing proteasome inhibitors have failed. Now scientists may have solved the mystery of these cells’ surprising hardiness. The key: Resistant cancer cells have shifted how and where they generate their energy. Using this new insight, researchers have identified a drug that resensitizes cancer cells to proteasome inhibitors and pinpointed a gene that is crucial for that susceptibility.
As cancer cells develop, they accrue multiple genetic alterations that allow the cells to quickly reproduce, spread and survive in distant parts of the body, and recruit surrounding cells and tissues to support the growing tumor. To perform these functions, cancer cells must produce high volumes of the proteins that support these processes. The increased protein production and numerous mutated proteins of cancer cells make them particularly dependent on the proteasome, which is the cell’s protein degradation machine. These huge protein complexes act as recycling machines, gobbling up unwanted proteins and dicing them into their amino acid building blocks, which can be reused for the production of other proteins.
Previously, researchers exploited cancer cells’ increased dependency on their proteasomes to develop anti-cancer therapies that inhibit the proteasomes’ function. Several distinct proteasome inhibitors have been developed, and when used in the lab, these proteasome inhibitor drugs are indeed highly effective at eradicating tumor cells. However, when administered to animal models or patients with cancer, such as multiple myeloma, proteasome inhibitors have limited efficacy and even initially vulnerable cancer cells quickly develop resistance to them. How do cancer cells so adroitly sidestep drugs that should kill them?
Photo by Erin Ashford Yale University Principal Investigator: David Spiegel Research Team: Prof. Jason Crawford, Nam Kim, Venkata Sabbasani, Matthew Streeter The long-lived collagen proteins that give structure to our arteries and other tissues are continuously exposed to blood sugar and other highly reactive molecules necessary for life. Occasionally, …Glucosepane Crosslinks and Undoing Age-Related Tissue Damage.
Unregulated cell division is a hallmark of cancer, and one of the key proteins involved in controlling cell division is called FoxM1. Abnormal activation of FoxM1 is a common feature of cancer cells and is correlated with poor prognosis, metastasis, and resistance to chemotherapy.
Now researchers at UC Santa Cruz have determined the structure of this protein—a kind of “master switch” for cell division—in its inactive or “off” conformation. This new understanding of the structure of FoxM1 could ultimately be used to design new drugs that stabilize the protein in its inactive state and thereby stop the uncontrolled proliferation of cancer cells.
Seth Rubin, professor of chemistry and biochemistry at UC Santa Cruz, explained that FoxM1 is a “transcription factor,” a protein that controls the activity of specific genes.
At low speed, it operates like a quadcopter, at high speed, it’s a jet-propelled, highly efficient supersonic aircraft whose entire body acts as a low-drag wing. Those are the claims of the Romanian creators of this flying saucer that’s designed to offer unprecedented aerial agility across a broad range of speeds.
By pieter spronck and jaap van den herik
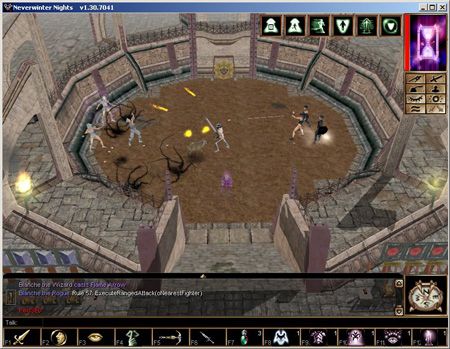
While the audiovisual qualities of games have improved significantly over the last twenty years, game artificial intelligence (AI) has been largely neglected. Since the turn of the century game development companies have discovered that nowadays it is the quality of the game AI that sets apart good games from mediocre ones. The Institute of Knowledge and Agent Technology (IKAT) of the Universiteit Maastricht examines methods to enhance game AI with machine learning techniques. Several typical characteristics of games, such as their inherent randomness, require novel machine learning approaches to allow them to deal with game AI.
Most commercial computer games contain computer-controlled agents that oppose the human player. ‘Game AI’ encompasses the decision-making capabilities of these agents. For implementing game AI, especially for complex games, developers usually resort to rule-based techniques in the form of scripts. Scripts have the advantage that they are easy to understand and can be used to implement fairly complex behaviour.