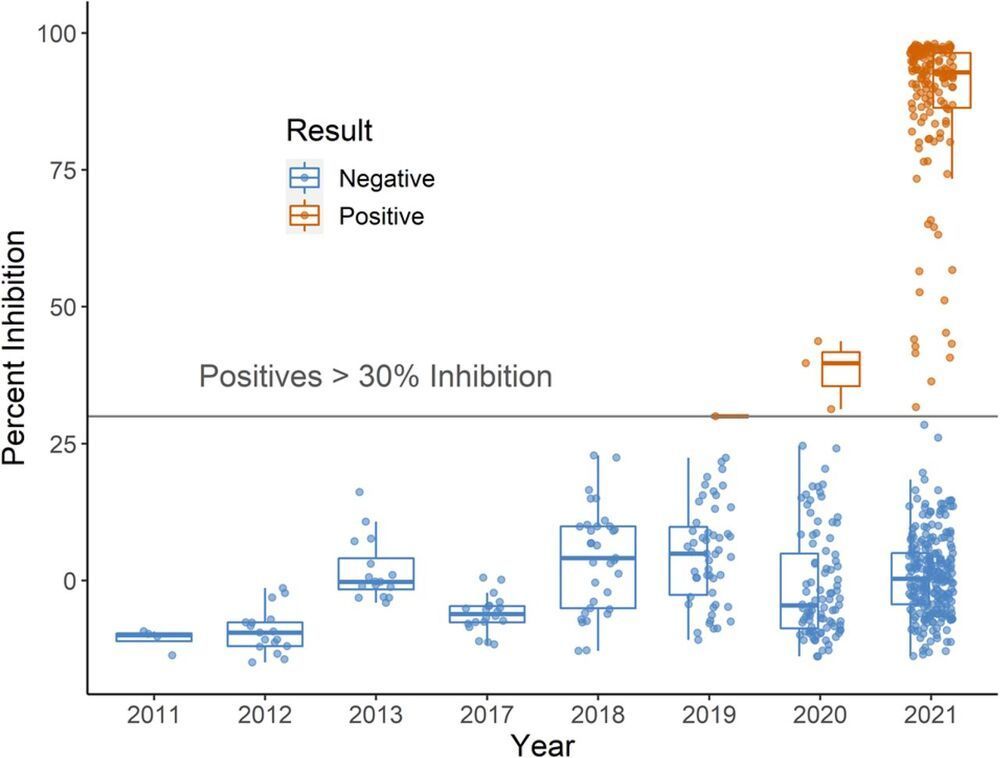
Widespread human SARS-CoV-2 infections combined with human-wildlife interactions create the potential for reverse zoonosis from humans to wildlife. We targeted white-tailed deer (Odocoileus virginianus) for serosurveillance based on evidence these deer have ACE2 receptors with high affinity for SARS-CoV-2, are permissive to infection, exhibit sustained viral shedding, can transmit to conspecifics, and can be abundant near urban centers. We evaluated 624 pre-and post-pandemic serum samples from wild deer from four U.S. states for SARS-CoV-2 exposure. Antibodies were detected in 152 samples (40%) from 2,021 using a surrogate virus neutralization test. A subset of samples was tested using a SARS-CoV-2 virus neutralization test with high concordance between tests. These data suggest white-tailed deer in the populations assessed have been exposed to SARS-CoV-2.
One-Sentence Summary Antibodies to SARS-CoV-2 were detected in 40% of wild white-tailed deer sampled from four U.S. states in 2021.
SARS-CoV-2, the virus that causes COVID-19 in humans, can infect multiple domestic and wild animal species (1 – 7). Thus, the possibility exists for the emergence of new animal reservoirs of SARS-CoV-2, each with unique potential to maintain, disseminate, and drive novel evolution of this virus. Of particular concern are wildlife species that are both abundant and live in close association with human populations (5).