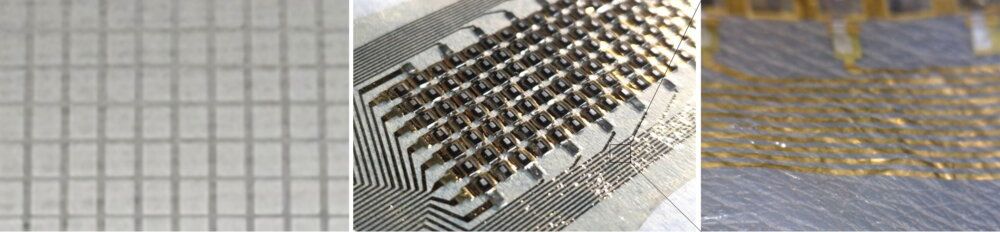
With the established success of flexible computer screen displays, many users are wondering how display technology will advance next. So far, free-form displays have grown popular as a next-generation product that offers both portability and high-resolution visuals.
While this technology is still quite new, a wealth of research already exists into the stretchable displays that make up free form displays, products that can stretch into any direction like rubber.
On June 4, 2021, research at Samsung appeared in the well-known journal Science Advances discussing a technology that bypasses the limitations of stretchable devices. The associated experiment showed stable performance even when the display was significantly elongated. As these products can already be used in existing semiconductor processes, Samsung researchers have high hopes about what this could mean for the commercialization and salability of stretchable devices.