Circa 2019
While we’ve seen plenty of runners propose postmarathon (or even, dare we say it, in the middle of a marathon), few have gone the extra mile—or in this case, the extra 100-plus miles—before popping the question.


You know you’re a little different when the family tags along for your run in an RV fully equipped for a multi-day road trip.
Have you tried pulling an all-nighter recently? It hurts. A once-common event in college – thanks to studying or partying or midnight hikes that turned into sunrise missions – becomes increasingly debilitating the older you get. It’s like your first run after some time off: You might feel okay doing it, but you’ll pay the next day.
Unless you’re the genetically blessed aberration that is Dean Karnazes, 53, one of the most well known runners of our time.
In 1992, after taking a 15-year break from running, it wasn’t enough for Karnazes’ first run to be 30 miles. Winning the infamous, 135-mile Badwater Ultramarathon across Death Valley in 120-degree heat didn’t cut it. Nor did pushing the opposite end of spectrum of human suffering by running a marathon to the South Pole, at-13-degrees F.

O,.o.
In a new study from the California Institute of Technology, experts have discovered that fruit flies can fly up to 15 kilometers in a single journey. This distance is the equivalent of the average human traveling over 10000 kilometers, or more than 6200 miles.
The record for the longest distance by a human was set in 2005, when an ultramarathon runner Dean Karnazes ran continuously for 80 hours over 350 miles – roughly 324000 times his body length. The Caltech study has found that fruit flies can travel up to six million times the length of their body.
The experts designed a series of “release and recapture” experiments involving hundreds of thousands of fruit flies under various wind conditions.

The CEO of Pfizer says that people who got the company’s version of the COVID-19 vaccine will likely need a booster shot within a year.
Albert Bourla made the announcement in an interview with CNBC correspondent Bertha Coombs that was filmed two weeks ago and released publicly on Thursday.
“Likely scenarios is there will likely be a need for a third dose somewhere between six and 12 months and then from there, there will be an annual vaccination,” says Bourla.
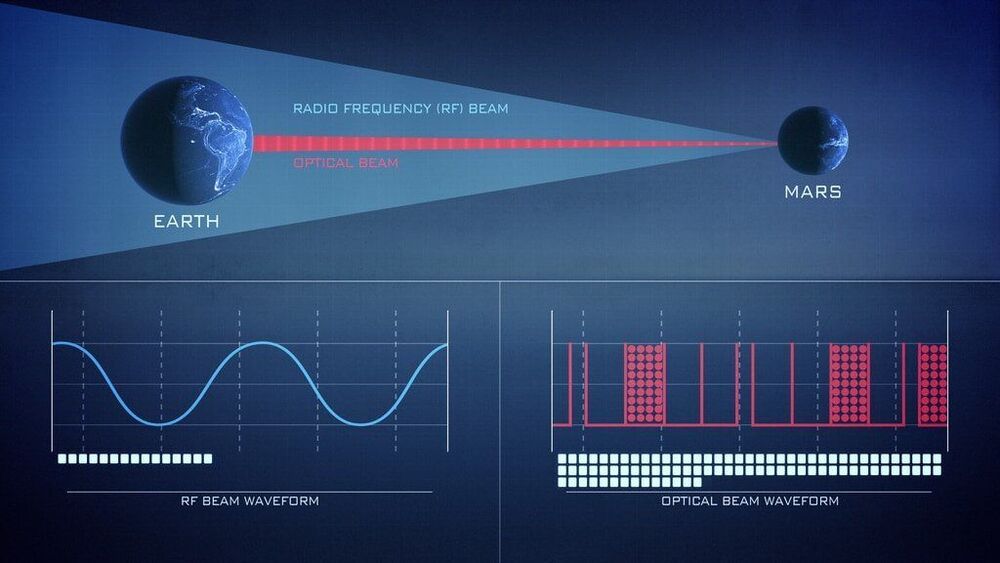
Launching this summer, NASA’s Laser Communications Relay Demonstration (LCRD) will showcase the dynamic powers of laser communications technologies. With NASA’s ever-increasing human and robotic presence in space, missions can benefit from a new way of “talking” with Earth.
Since the beginning of spaceflight in the 1950s, NASA missions have leveraged radio frequency communications to send data to and from space. Laser communications, also known as optical communications, will further empower missions with unprecedented data capabilities.
Scientists from the Institute of Scientific and Industrial Research at Osaka University have used machine-learning methods to enhance the signal-to-noise ratio in data collected when tiny spheres are passed through microscopic nanopores cut into silicon substrates. This work may lead to much more sensitive data collection when sequencing DNA or detecting small concentrations of pathogens.
Miniaturization has opened the possibility for a wide range of diagnostic tools, such as point-of-care detection of diseases, to be performed quickly and with very small samples. For example, unknown particles can be analyzed by passing them through nanopores and recording tiny changes in the electrical current. However, the intensity of these signals can be very low, and is often buried under random noise. New techniques for extracting the useful information are clearly needed.
Now, scientists from Osaka University have used deep learning to “denoise” nanopore data. Most machine learning methods need to be trained with many “clean” examples before they can interpret noisy datasets. However, using a technique called Noise2Noise, which was originally developed for enhancing images, the team was able to improve resolution of noisy runs even though no clean data was available. Deep neural networks, which act like layered neurons in the brain, were utilized to reduce the interference in the data.

Three billion years ago, light first zipped through chlorophyll within tiny reaction centers, the first step plants and photosynthetic bacteria take to convert light into food.
Heliobacteria, a type of bacteria that uses photosynthesis to generate energy, has reaction centers thought to be similar to those of the common ancestors for all photosynthetic organisms. Now, a University of Michigan team has determined the first steps in converting light into energy for this bacterium.
“Our study highlights the different ways in which nature has made use of the basic reaction center architecture that emerged over 3 billion years ago,” said lead author and U-M physicist Jennifer Ogilvie. “We want to ultimately understand how energy moves through the system and ends up creating what we call the ‘charge-separated state.’ This state is the battery that drives the engine of photosynthesis.”
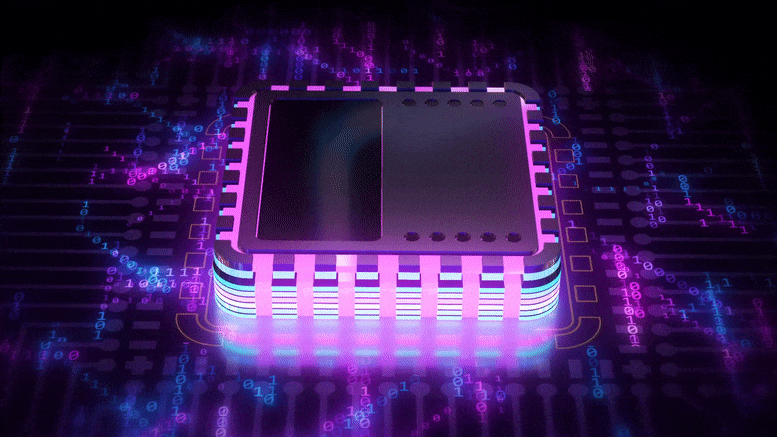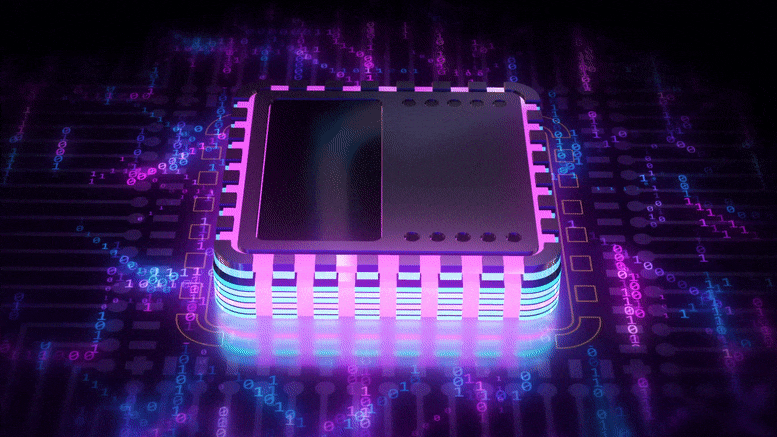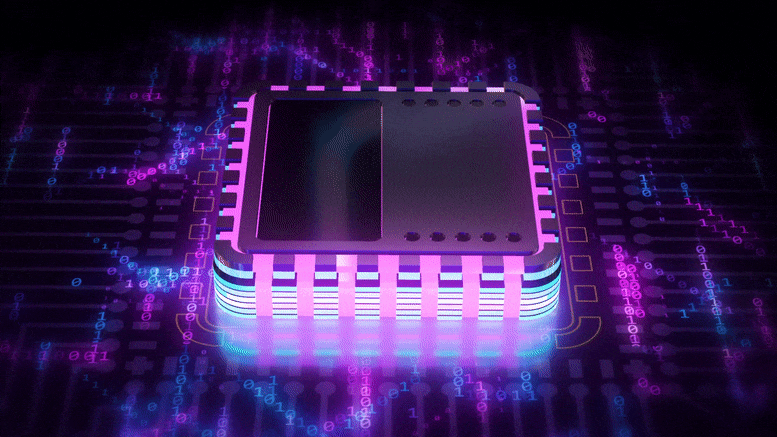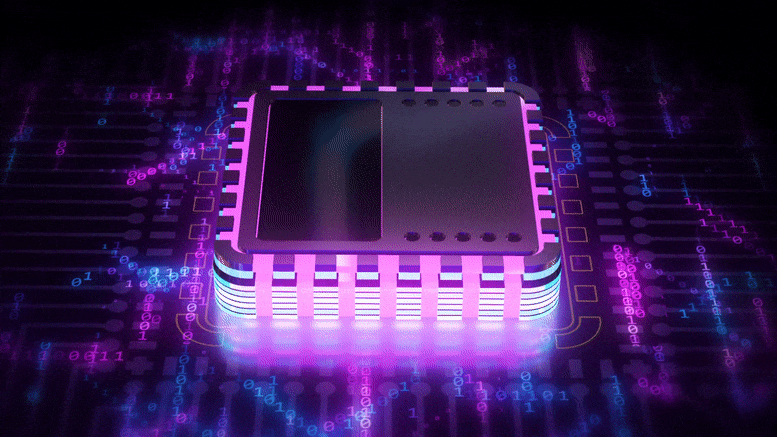
Harnessing the Hum of Fluorescent Lights for More Efficient Computing
The property that makes fluorescent lights buzz could power a new generation of more efficient computing devices that store data with magnetic fields, rather than electricity.
A team led by University of Michigan researchers has developed a material that’s at least twice as “magnetostrictive” and far less costly than other materials in its class. In addition to computing, it could also lead to better magnetic sensors for medical and security devices.