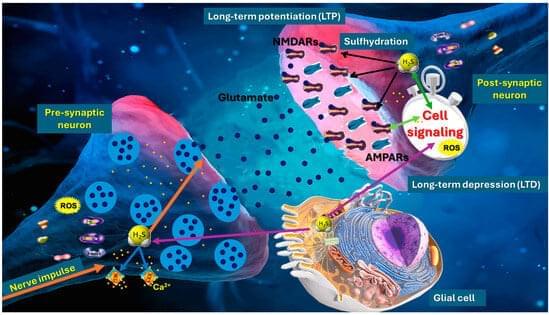
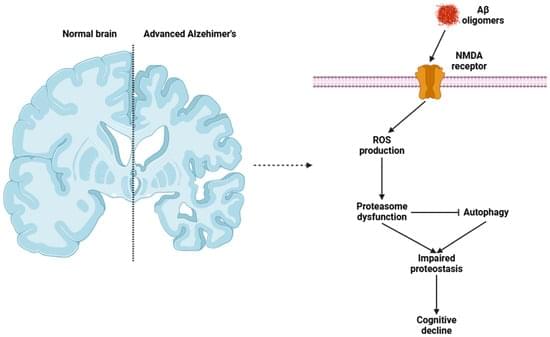
Hydrogen sulfide (H2S) has emerged as a pivotal gaseous transmitter in the central nervous system, influencing synaptic plasticity, learning, and memory by modulating various molecular pathways. This review examines recent evidence regarding how H2S regulates NMDA receptor function and neurotransmitter release in neuronal circuits. By synthesizing findings from animal and cellular models, we investigate the impacts of enzymatic H2S production and exogenous H2S on excitatory synaptic currents, long-term potentiation, and intracellular calcium signaling. Data suggest that H2S interacts directly with NMDA receptor subunits, altering receptor function and modulating neuronal excitability. Simultaneously, H2S promotes the release of neurotransmitters such as glutamate and GABA, shaping synaptic dynamics and plasticity.
Hydrogen Sulfide (H2S- or H2Sn-Polysulfides) in Synaptic Plasticity: Modulation of NMDA Receptors and Neurotransmitter Release in Learning and Memory
Posted in futurism | Leave a Comment on Hydrogen Sulfide (H2S- or H2Sn-Polysulfides) in Synaptic Plasticity: Modulation of NMDA Receptors and Neurotransmitter Release in Learning and Memory