
Get the latest international news and world events from around the world.



Russian forces seize control of Chernobyl nuclear plant, Ukrainian official says
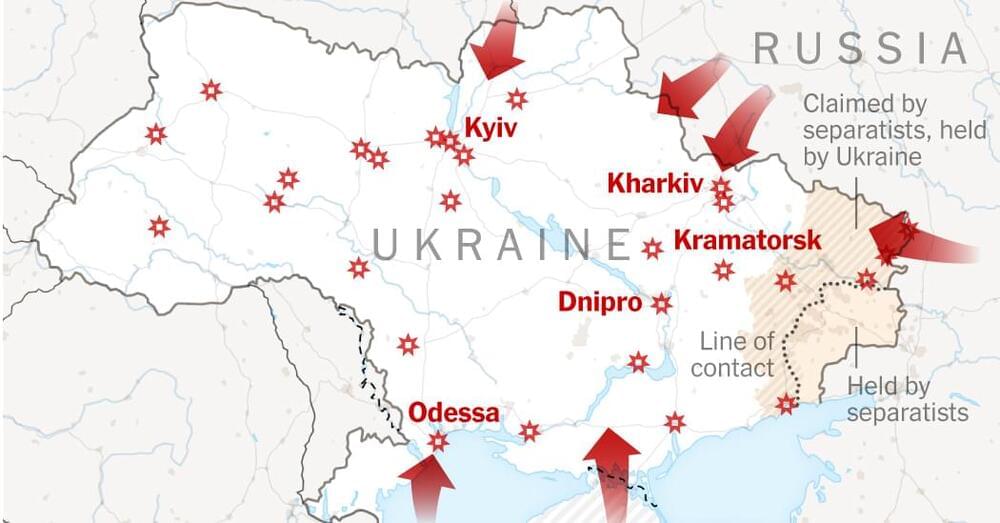
Russian forces have seized control of the Chernobyl power plant in northern Ukraine, the site of the world’s worst nuclear disaster, according to the agency that manages the area.
Troops overran the plant on the first day of Russia’s multi-pronged invasion of Ukraine, a spokesperson for the State Agency of Ukraine on Exclusion Zone Management, Yevgeniya Kuznetsovа, told CNN.
“When I came to the office today in the morning (in Kyiv), it turned out that the (Chernobyl nuclear power plant) management had left. So there was no one to give instructions or defend,” she said.

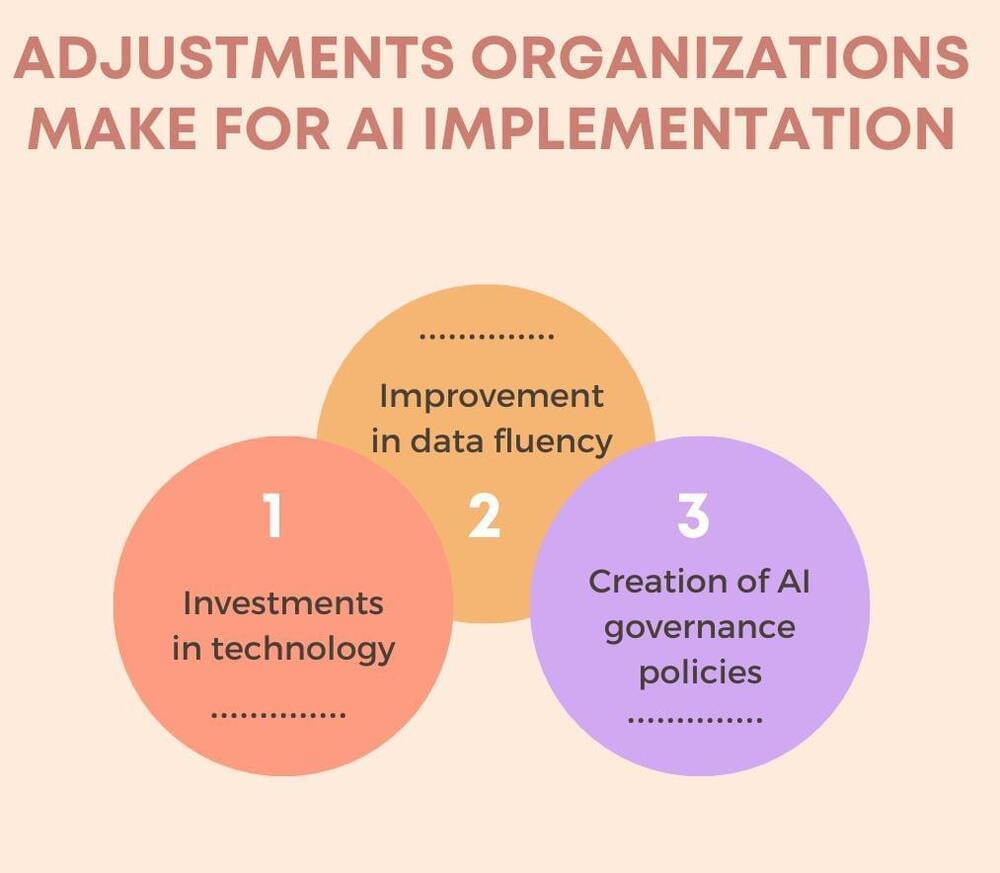
How the tech industry is responding to Russia’s invasion of Ukraine
The invasion was met with sharp rebuke from the United States, the European Union and NATO allies, with broad, unprecedented financial and diplomatic sanctions promised against Russia, sanctions that are likely to affect business, trade and finance across the region.
The impacts of the invasion are also, undoubtedly, being felt across Ukraine’s wider tech ecosystem, which includes not only hundreds of startups and larger tech firms, but also research and development offices for some of the world’s biggest technology brands.
As the situation on the ground changes rapidly over the next few hours and days, TechCrunch will continue to bring news and analysis on how the conflict unfolds across the tech and startup community.
Welp, Now We Have Robo-Dogs With Sniper Rifles
The “Special Purpose Unmanned Rifle” has materialized from your Black Mirror nightmares.
Science fiction has seeped into science reality this week, as a robotics company showed off its sniper rifle-equipped robo-dog at the Association of the U.S. Army’s annual convention in Washington, D.C.
✈︎ Don’t miss our best-in-class military news. Join our squadron.