In nature and technology, crystallization plays a pivotal role, from forming snowflakes and pharmaceuticals to creating advanced batteries and desalination membranes. Despite its importance, crystallization at the nanoscale is poorly understood, mainly because observing the process directly at this scale is exceptionally challenging. My research overcame this hurdle by employing state-of-the-art computational methods, allowing them to visualize atomic interactions in unprecedented detail.
Published in Chemical Science, my research has uncovered new details about how salt crystals form in tiny nanometer-sized spaces, which could pave the way for advanced materials and improved electrochemical technologies.
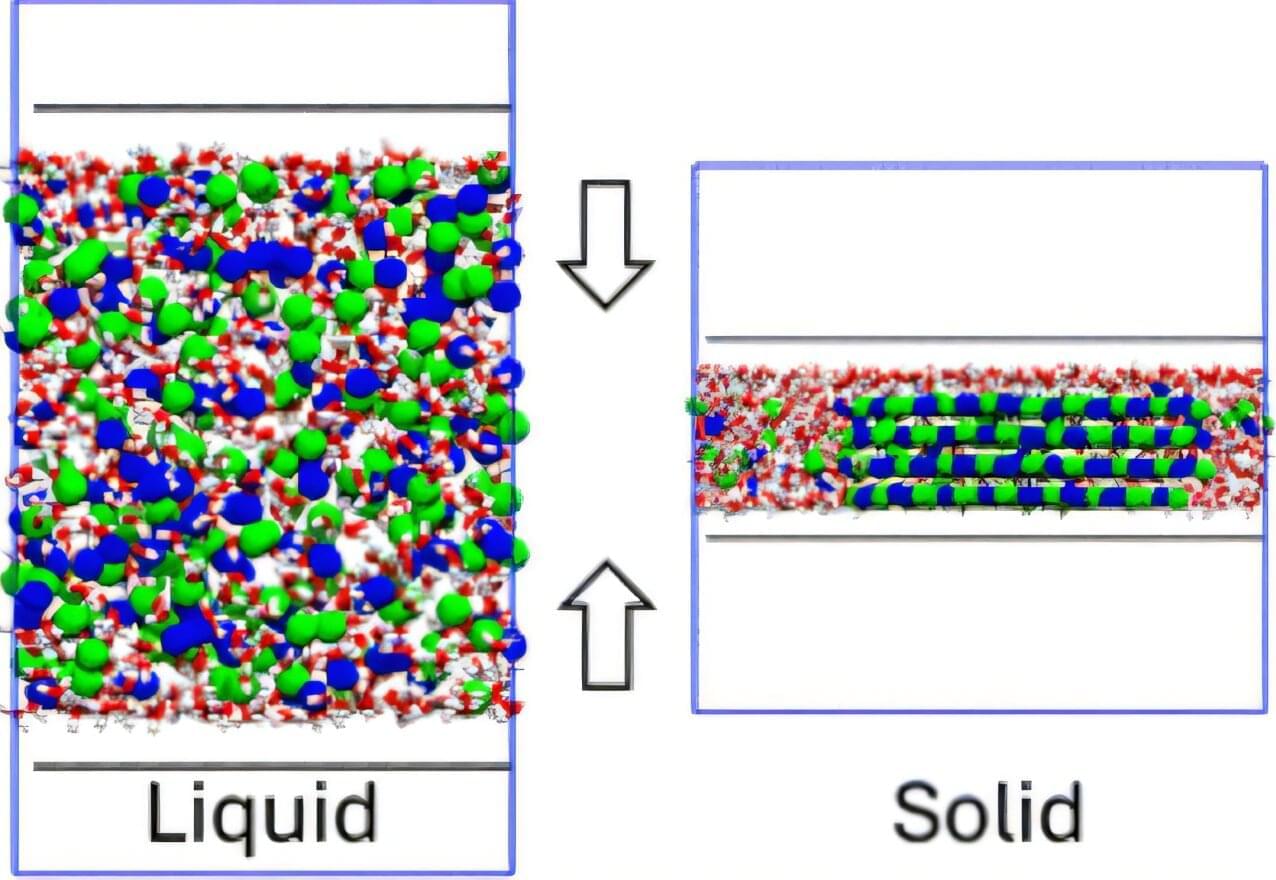
This research used sophisticated molecular dynamics simulations enhanced by cutting-edge machine learning techniques to study how sodium chloride (NaCl), common table salt, crystallizes when confined between two graphene sheets separated by just a few billionths of a meter. These extreme conditions, known as nano-confinement, drastically alter how molecules behave compared to bulk, everyday conditions.