The company’s engineers said that the new device may not be slated for use with any of the current IBM Quantum processors but that building it taught them important lessons on how to overcome these challenges.
Get the latest international news and world events from around the world.

Alien Impostors & Doppelgangers
Start listening with a 30-day Audible trial and your first audiobook is free. Visit http://www.audible.com/isaac or text “isaac” to 500–500.
The idea that some mimic might steal your identity and replace you, or takeover your mind, is terrifying. But could we encounter aliens that were able to do this?
Visit our Website: http://www.isaacarthur.net.
Support us on Patreon: https://www.patreon.com/IsaacArthur.
Support us on Subscribestar: https://www.subscribestar.com/isaac-arthur.
Facebook Group: https://www.facebook.com/groups/1583992725237264/
Reddit: https://www.reddit.com/r/IsaacArthur/
Twitter: https://twitter.com/Isaac_A_Arthur on Twitter and RT our future content.
SFIA Discord Server: https://discord.gg/53GAShE
Listen or Download the audio of this episode from Soundcloud: Episode’s Audio-only version: https://soundcloud.com/isaac-arthur-148927746/alien-impostors-doppelgangers.
Episode’s Narration-only version: https://soundcloud.com/isaac-arthur-148927746/alien-impostor…ation-only.
Credits:
Alien Civilizations: Alien Impostors & Doppelgangers.
Science & Futurism with Isaac Arthur.
Episode 359a, September 11, 2022
Written, Produced & Narrated by Isaac Arthur.
Editors:
David McFarlane.
Graphics:
How Did Life Begin?
Researched and Written by Leila Battison.
Narrated and Edited by David Kelly.
Script Edited by Pete Kelly.
Art by Khail Kupsky.
Thumbnail Art by Ettore Mazza.
If you like our videos, check out Leila’s youtube channel:
https://www.youtube.com/channel/UCXIk7euOGq6jkptjTzEz5kQ
References for the video:
https://archive.org/details/molecularorigins0000brac/page/2/mode/2up https://www.springer.com/cda/content/document/cda_downloaddo…p173822459 https://www.wired.com/2014/06/fantastically-wrong-how-to-gro…-are-wrong https://penelope.uchicago.edu/Thayer/E/Journals/TAPA/51/Spontaneous_Generation*.html#note52 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2926753/
Thanks to:

How Did Human Beings Acquire the Ability to do Math?
(October 29, 2012) Keith Devlin concludes the course by discussing the development of mathematical cognition in humans as well as the millennium problems.
Originally presented in the Stanford Continuing Studies Program.
Stanford University:
Stanford Continuing Studies Program:
https://continuingstudies.stanford.edu/
Stanford University Channel on YouTube:
Scientists Are Working on a Gene-Hacking Drug That Could Treat Baldness
Using gene modification techniques, a team of researchers have come up with a new treatment for balding, Wired reports — a condition experienced to varying degrees by two-thirds of American men by age 35.
The team, associated with the University of California, Irvine and a biotech company called Amplifica, believes they’ve identified the signaling pathway that drive hair growth to find new ways to stop stem cells from giving up on producing hair follicles.
Experiments with mice, as detailed in a new paper published in the journal Developmental Cell last month, have been promising. The mice were genetically modified to have the hair growth signaling pathway turned on permanently.
Trust in AI-based preventive healthcare low among users
Artificial Intelligence (AI) can perform preventive healthcare activities such as health screening, routine check-up and vaccination with expert-level accuracy that can turn out to be cost-effective in the long run. Yet, a new research found that individuals show less trust in preventive care interventions suggested by AI than when the same interventions are prompted by human health experts.
The researchers at Nanyang Technological University (NTU) Singapore studied 15,000 users of a health mobile application and found that emphasising the involvement of a human health expert in an AI-suggested intervention could improve its acceptance and effectiveness.
These findings suggest that the human element remains important even as the healthcare sector increasingly adopts AI to screen, diagnose and treat patients more efficiently. The findings could also contribute to the design of more effective AI-prompted preventive care interventions, said the researchers.
DHEA-S Is A Weakness In My Data: Blood Test #5 in 2022
Join us on Patreon!
https://www.patreon.com/MichaelLustgartenPhD
Quantify Discount Link (At-Home Blood Testing)
https://getquantify.io/mlustgarten.
TruDiagnostic Discount Link (Epigenetic Testing)
CONQUERAGING!
https://bit.ly/3Rken0n.
Bristle Discount Link (Oral microbiome quantification):
ConquerAging15
https://www.bmq30trk.com/4FL3LK/GTSC3/
Cronometer Discount Link (Daily diet tracking):
https://shareasale.com/r.cfm?b=1390137&u=3266601&m=61121&urllink=&afftrack=
Support the channel with Buy Me A Coffee!
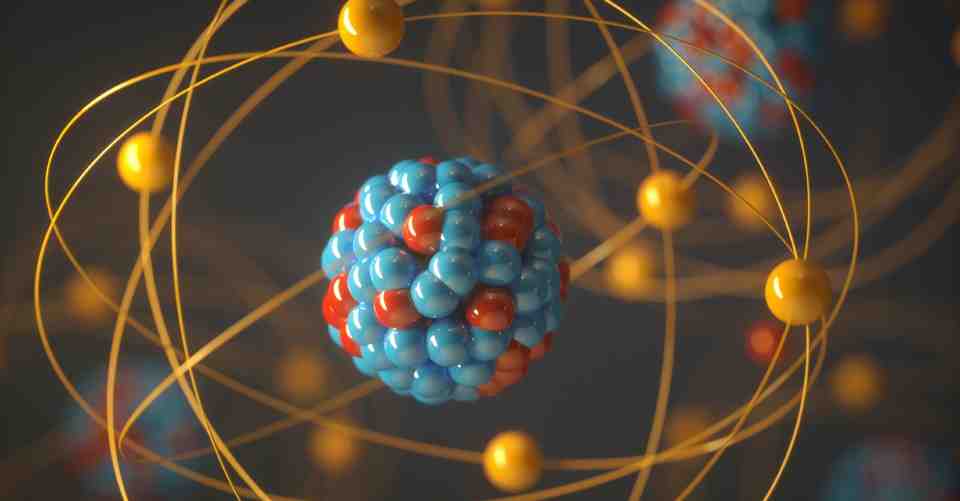
What is the Standard Model?
The Standard Model is our best theory for how the universe operates, but there are some missing pieces that physicists are struggling to find.
The Standard Model of physics is the theory of particles, fields and the fundamental forces that govern them.
It tells us about how families of elementary particles group together to form larger composite particles, and how one particle can interact with another, and how particles respond to the fundamental forces of nature. It has made successful predictions such as the existence of the Higgs boson, and acts as the cornerstone for theoretical physics.