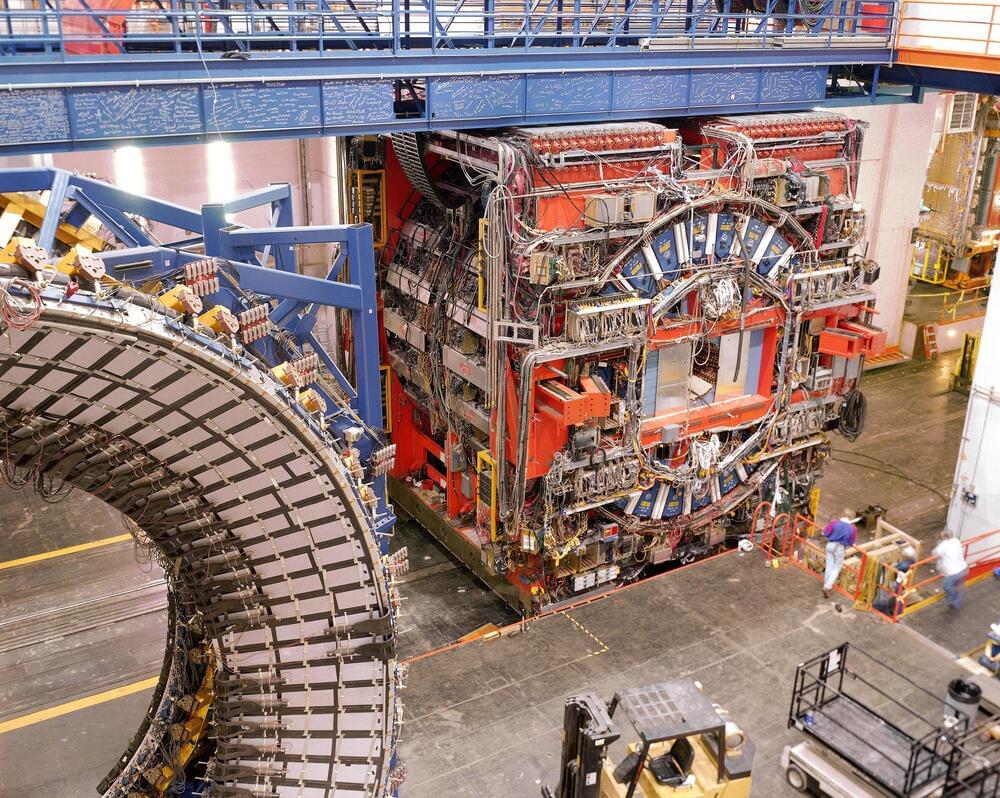
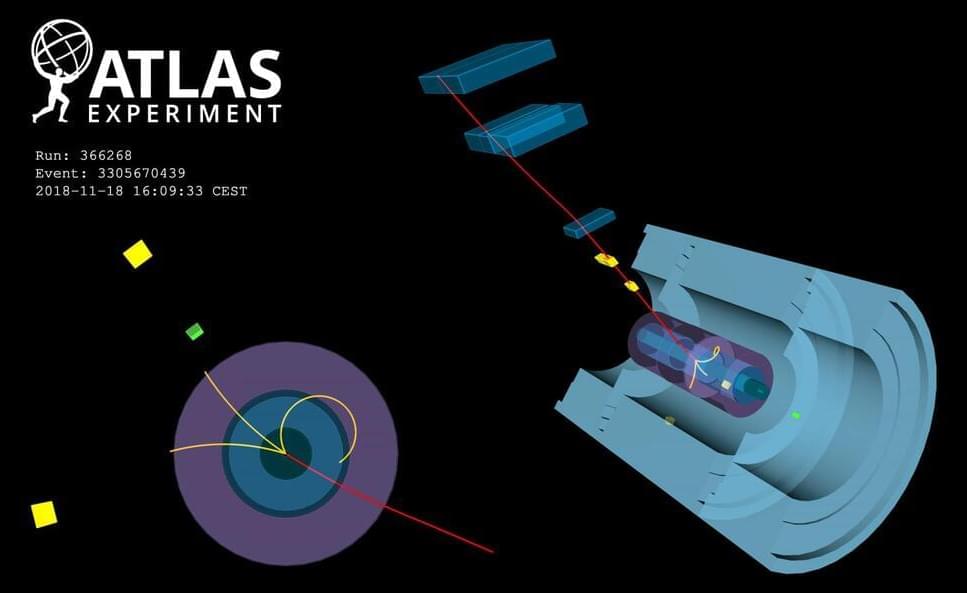
In particle physics, data long outlives the detectors that generate it. A decade ago the 4,100-metric-ton Collider Detector at Fermilab (CDF) reached the end of its life and was shut down, stripped of its parts for use in other experiments. Now a fresh analysis of old CDF data has unearthed a stunning discrepancy in the mass of an elementary particle, the W boson, that could point the way to new, as yet undiscovered particles and interactions.
The W boson is massive, some 80 times heavier than a proton. Crucially, the W boson is responsible for certain forms of radioactive decay, allowing neutrons to convert into protons. Because its mass is constrained by (and itself constrains) many other particles and parameters within the Standard Model—particle physicists’ theory of fundamental particles and how they behave—the W boson has become a target for researchers seeking to understand where and how their best theories fail.
Although physicists have long known the W boson’s approximate mass, they still do not know it exactly. Plugging data into the Standard Model framework, however, predicts that the so-called W mass should be 80,357 mega-electron-volts (MeV), plus or minus 6 MeV. (One MeV is about twice the mass-energy contained within a single electron.) But in a new analysis published on Thursday in Science, physicists on the CDF collaboration have instead found the W boson mass to be 80,433.5 ± 9.4 MeV. The new measurement, which is more precise than all previous measurements combined, is nearly 77 MeV higher than the Standard Model’s prediction. Although these numbers differ by only about one part in 1,000, the uncertainties for each are so minuscule that even this small divergence is of enormous statistical significance—it is exceedingly unlikely to be an illusion produced through sheer chance. The well-studied W boson, it seems, still holds plenty of secrets about the workings of the subatomic world—or at least about how we investigate it. Taken by surprise, particle physicists are only beginning to grapple with the implications.