In recent years, engineers have been working to develop increasingly sophisticated and smaller electronic components that could power the devices of the future. This includes thin and stretchable components that could be easily worn on the skin or implanted inside the human body.
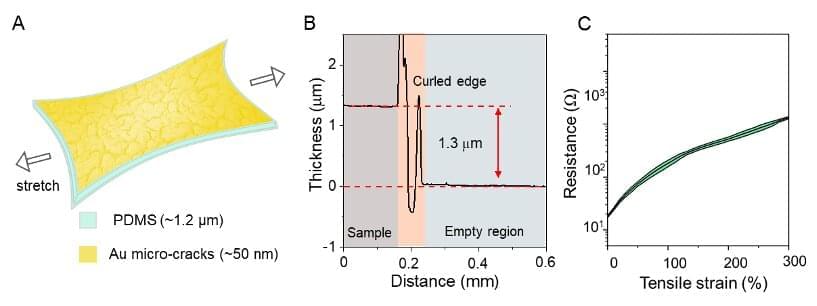
Researchers at RIKEN, Nanyang Technological University, National University of Singapore, University of Tokyo, and other institutes in Japan, Singapore and China have recently realized a new, elastic electrical conductor that is 1.3-micrometers thin. This conductor, introduced in a paper published in Nature Electronics, could advance the development of both wearable and implantable sensors.
“Ultrathin electronic devices can form a conformal interface with curved surfaces, are not perceivable by human when wearing, and do not induce strong foreign body rejection (FBR) when implanted in animals,” Zhi Jiang, one of the researchers who carried out the study, told TechXplore.