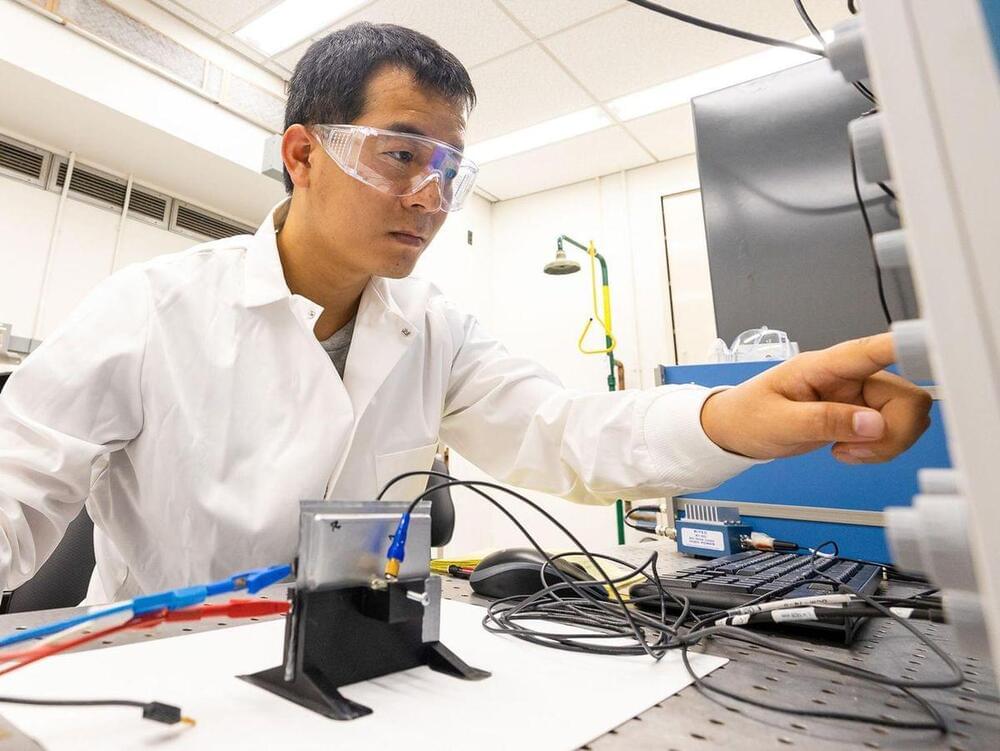
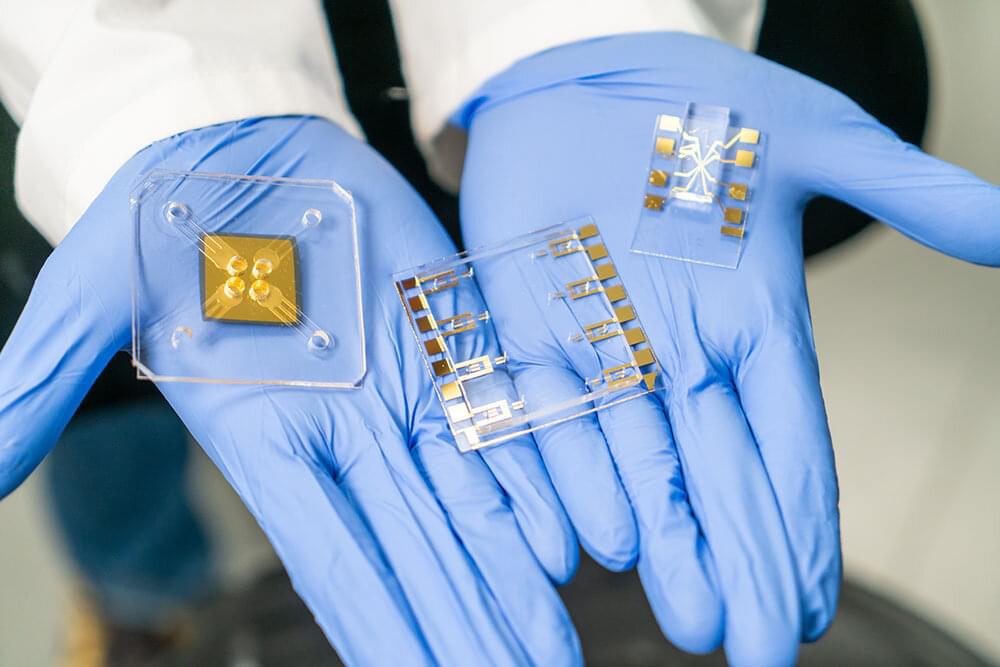
Ultrasound sensors as small as a thumbnail can scan lithium-ion batteries to check their charge, health, and safety, a new study finds.
The findings suggest that ultrasound—that is, sound waves at frequencies higher than human hearing can detect—might one day help electric vehicles better estimate how much charge remains in their batteries. This approach might also help detect unstable batteries on the verge of disaster, quickly test battery quality during manufacturing, and identify which used batteries are healthy enough to be resold to reduce waste, says study lead author Hongbin Sun, an ultrasonic engineer at the Oak Ridge National Laboratory, in Tennessee.
Estimating how much charge is left in a commercial lithium-ion battery is currently a challenging task. For instance, electric vehicles typically experience an uncertainty of about 10 percent when estimating battery charge. This in turn reduces their driving range by about 10 percent, to ensure that they stay within their batteries’ safety margins.