NASA published a video from its Artemis I Orion spacecraft, when it saw Earth disappear behind the Moon at the halfway point of its mission.



I’m so waiting for it! Let the new year go along the path of compassion.
For all who would like to stop animall suffer and still have their favourite meal. There is still a lot of work to be done to make this happen, but much has already been done.
A California-based lab-grown meat start-up received the first green light for such products from the U.S. food safety agency on No. 16, although the product still has more hurdles to clear before being sold to consumers.

Year 2022 face_with_colon_three
From the rise of ‘synthetic creativity’ to a study of doppelgängers and a road trip to the edge of the universe, these were the stories we loved in 2022.

Year 2022 face_with_colon_three
Since the discovery of black holes, they have inspired images of the universe’s extremities in both scientists and storytellers. Their immense gravity — sucking in any matter and light unfortunate enough to come within grabbing distance — conjures images of crushing death and infinite possibility.
That same gravity, however, creates a well which consumes indiscriminately and from whence nothing can ever emerge. The only trouble is that isn’t the case. Among Stephen Hawking’s many accomplishments was the discovery that black holes actually radiate very slowly and will eventually evaporate. This discovery, while enough to make Hawking famous, threw a wrench in contemporary astrophysics by creating a paradox.
If a black hole compresses into a singular point at its center and is surrounded by a gravitational event horizon, then the radiation emerging from the horizon is necessarily separate from the matter in the middle. In short, that radiation contains none of the information related to the matter which fell inside. If that’s true, then causality essentially breaks down around a black hole and physicists didn’t like that one bit.

Year 2011 face_with_colon_three
It was only a year ago that ACell’s “miracle powder” was sprinkled on amputated fingers and shown to stimulate the regeneration of fingertips. The world was both awed and skeptical of the powder’s regenerative power, touting that it would revolutionize regenerative medicine or calling it was quack science.
A fingertip is one thing. A thigh, quite another.
After losing most of his thigh muscle in a battlefield explosion, one marine was given a second chance when another such miracle powder caused much of his thigh to grow back. It’s not only a wonderful feel-good story, but demonstrating that the same substance can grow back different tissues suggests that we may have only seen a small part of its full regenerative potential.
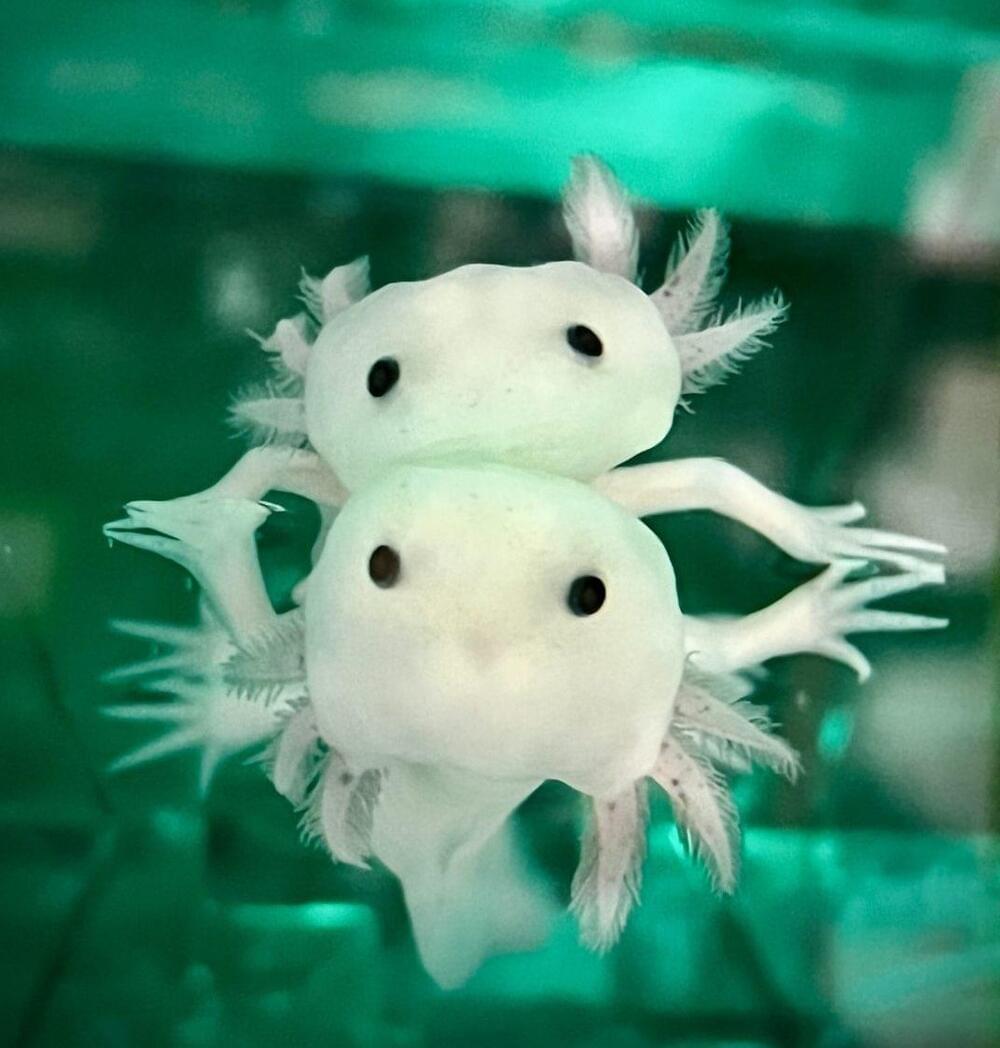
Year 2022 face_with_colon_three
One day, humans might be able to regrow body parts, regenerate tissue damaged due to disease, and even sprout missing limbs.
While it’s still in the realm of science fiction today, advanced tissue and limb regeneration might be our future thanks to the foundation being laid by scientists like assistant professor James Godwin of Mount Desert Island Biological Laboratory in Maine.
Godwin is studying a Mexican salamander called Ambystoma mexicanum, or axolotl — a name given to it by the Aztecs.

Year 2021 face_with_colon_three
In recent years, the use of deep learning in language models has gained much attention. Some research projects claim that they can generate text that can be interpreted as human writing, enabling new possibilities in many application areas. Among the different areas related to language processing, one of the most notable in applying this type of modeling is programming languages. For years, the machine learning community has been research ing this software engineering area, pursuing goals like applying different approaches to auto-complete, generate, fix, or evaluate code programmed by humans. Considering the increasing popularity of the deep learning-enabled language models approach, we found a lack of empirical papers that compare different deep learning architectures to create and use language models based on programming code.

Year 2021 face_with_colon_three
“This [study] shows us the evolution of the QGP and eventually [could] suggest how the early universe evolved in the first microsecond after the Big Bang,” said co-author You Zhou, an associate professor at the Niels Bohr Institute, University of Copenhagen in Denmark in an official statement.
“First the plasma that consisted of quarks and gluons was separated by the hot expansion of the universe. Then the pieces of quark reformed into so-called hadrons. A hadron with three quarks makes a proton, which is part of atomic cores. These cores are the building blocks that constitutes earth, ourselves and the universe that surrounds us.”
Following the chaotic Big Bang event, the universe was believed to be a violent soup of energy prior to it quickly expanding in a process known as inflation, where the infant universe cools to a point when matter is eventually formed.

The efficacy of implanted biomaterials is largely dependent on the response of the host’s immune and stromal cells. Severe foreign body response (FBR) can impede the integration of the implant into the host tissue and compromise the intended mechanical and biochemical function. Many features of FBR, including late-stage fibrotic encapsulation of implants, parallel the formation of fibrotic scar tissue after tissue injury. Regenerative organisms like zebrafish and salamanders can avoid fibrosis after injury entirely, but FBR in these research organisms is rarely investigated because their immune competence is much lower than humans. The recent characterization of a regenerative mammal, the spiny mouse (Acomys), has inspired us to take a closer look at cellular regulation in regenerative organisms across the animal kingdom for insights into avoiding FBR in humans.