In computing, a handshake is a signal between two devices or programs, used to, e.g., authenticate, coordinate. An example is the handshaking between a hypervisor and an application in a guest virtual machine.


View recent discussion. Abstract: The remarkable zero-shot capabilities of Large Language Models (LLMs) have propelled natural language processing from task-specific models to unified, generalist foundation models. This transformation emerged from simple primitives: large, generative models trained on web-scale data. Curiously, the same primitives apply to today’s generative video models. Could video models be on a trajectory towards general-purpose vision understanding, much like LLMs developed general-purpose language understanding? We demonstrate that Veo 3 can solve a broad variety of tasks it wasn’t explicitly trained for: segmenting objects, detecting edges, editing images, understanding physical properties, recognizing object affordances, simulating tool use, and more.
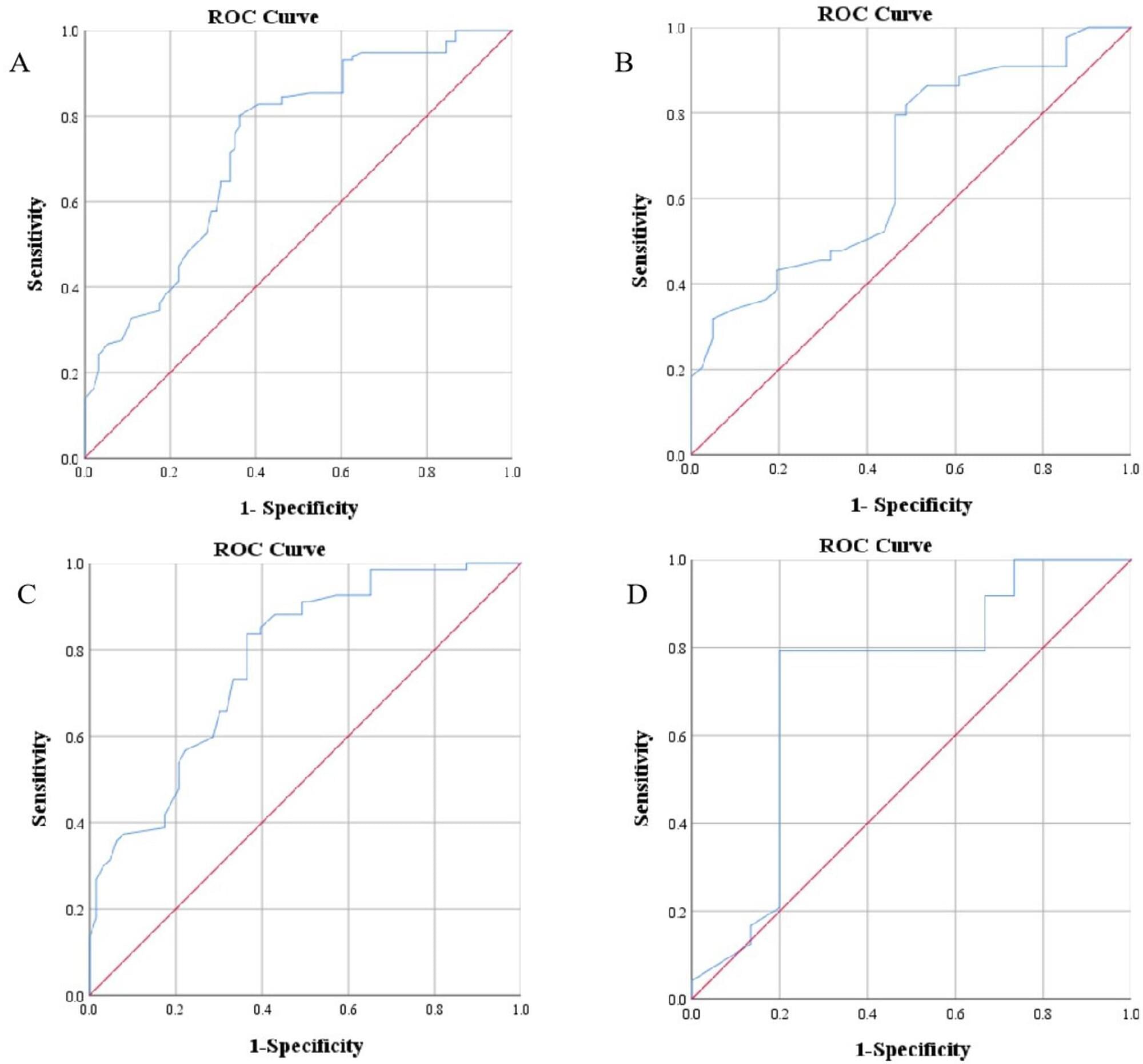
Early oral propranolol for infantile hemangioma (IH) can improve the success rate. However, few studies have been conducted on the specific age threshold for initiating treatment with propranolol. This study aimed to determine the cutoff value of treatment success for IH with oral propranolol.
This was a retrospective study involving 207 patients with IH and clinical data. The primary outcome measure was treatment success with oral propranolol at months 6 to 12.
Multivariate analysis showed that age at treatment initiation (P 0.001), high-risk IH (P = 0.002), and segmental IH (P = 0.012) were independent risk factors for treatment unsuccess with oral propranolol at months 6 to 12. Receiver operating characteristic (ROC) curve analysis indicated that the cutoff value for age at treatment initiation was 69.5 days for all patients, 65.5 days for patients with segmental IH or high-risk IH, and 93.5 days for patients with non-high-risk and nonsegmental IH. Patients who started treatment before 69.5 days had a success rate of 73.8%, which was higher than the 28.4% success rate of patients who started treatment after 69.5 days. The median time to treatment success for patients who started treatment before 69.5 days was 11.5 months, which was shorter than the 15 months for patients who started treatment after 69.5 days.
This video is essential viewing to get a good overall feel for where we are now — and where we’re going — with AI, AGI, and the risks we face because of both. It’s a fantastically entertaining watch too!
Would AI hurt us and cause human extinction? Use code insideai at https://incogni.com/insideai to get an exclusive 60% off.
Expert opinion from Geoffrey Hinton, Ilya Sutskever, Max Tegmark.
Google Gemini, Open AI Chat GPT, Deepseek, Grok, Google Gemini.
0:00 — 0:38 — Intro.
0:39 — 0:59 — AI style.
1:00 — 1:19 — Max Chat GPT
1:20 — 1:36 — AI Girlfriend.
1:37 — 1:48 — Jailbroken AI
1:49 — 2:29 — AI Risk Questions 1
2:30 — 2:55 — Would AI turn on us?
2:56 — 3:20 — Intense AI Girlfriend.
3:21 — 3:44 — Jailbroken AI
3:45 — 4:57 — Can we Trust AI?
4:58 — 5:54 — Jailbreaking Max.
5:55 — 6:14 — AI Girlfriend.
6:15 — 6:42 — Jailbroken Max.
6:43 — 7:06 — Girlfriend in car.
7:07 — 7:45 — AI Risk Questions 2
7:46 — 9:48 — Incogni Ad.
9:49 — 10:27 — AI Girlfriend meets Max.
10:28 — 10:57 — Jailbroken Max.
10:58 — 11:37 — AI Risk Questions Pt 3
11:38 — 12:11 — AI Girlfriends good for us?
12:12 — 12:42 — Resetting Chat GPT
12:43 — 13:48 — Crazy AI Predictions.
13:49 — 14:50 — AI Safety.
14:51 — 15:09 — Ilya Sutskever.
15:10 — 15:26 — Geoffrey Hinton.
15:27 — 15:45 — Max Tegmark.
15:46 — 16:00 — AI Final Thought.
#artificialintelligence #AI #chatbot #superintelligence #aigirlfriend #insideai
Gravity has always shackled industry, but orbit frees us to build in ways Earth never allowed. This episode explores the rise of orbital foundries and the industries they unlock.
Support us on Patreon: / isaacarthur.
Support us on Subscribestar: https://www.subscribestar.com/isaac-a… Group: / 1,583,992,725,237,264 Reddit:
/ isaacarthur Twitter:
/ isaac_a_arthur on Twitter and RT our future content. SFIA Discord Server:
/ discord Credits: Orbital Foundries & Zero-G Manufacturing: Building in Space Written, Produced & Narrated by: Isaac Arthur Graphics: Jeremy Jozwik, Ken York, Udo Schroeter Select imagery/video supplied by Getty Images Music Courtesy of Aerium, Stellardrone, Chris Zabriskie, and Epidemic Sound http://epidemicsound.com/creator Chapters 0:00 Intro 1:24 Why Manufacture in Space? 6:15 Near-Term Zero-G Manufacturing Efforts 12:08 Mid-Term: Orbital Foundries and Industrial Expansion 18:47 Patreon 19:10 Long-Term: True Orbital Foundries and Gigascale Production 25:38 Far Future: Manufacturing Planets, Stars, and Beyond 30:10 From Tiny Threads to Stellar Foundries.
Facebook Group: / 1583992725237264
Reddit: / isaacarthur.
Twitter: / isaac_a_arthur on Twitter and RT our future content.
SFIA Discord Server: / discord.
Credits:
Orbital Foundries & Zero-G Manufacturing: Building in Space.
Written, Produced & Narrated by: Isaac Arthur.
Graphics: Jeremy Jozwik, Ken York, Udo Schroeter.
Select imagery/video supplied by Getty Images.
Music Courtesy of Aerium, Stellardrone, Chris Zabriskie, and Epidemic Sound http://epidemicsound.com/creator.
Chapters.
0:00 Intro.
1:24 Why Manufacture in Space?
6:15 Near-Term Zero-G Manufacturing Efforts.
12:08 Mid-Term: Orbital Foundries and Industrial Expansion.
18:47 Patreon.
19:10 Long-Term: True Orbital Foundries and Gigascale Production.
25:38 Far Future: Manufacturing Planets, Stars, and Beyond.
30:10 From Tiny Threads to Stellar Foundries.

University of Warwick astronomers have uncovered the chemical fingerprint of a frozen, water-rich planetary fragment being consumed by a white dwarf star outside our solar system.
In our solar system, it is thought that comets and icy planetesimals (small solid objects in space) were responsible for delivering water to Earth. The existence of these icy objects is a requirement for the development of life on other worlds, but it is incredibly difficult to identify them outside our solar system as icy objects are small, faint and require chemical analysis.
In a study published in Monthly Notices of the Royal Astronomical Society, astronomers from Warwick, Europe and the US have found strong evidence that icy, volatile-rich bodies—capable of delivering water and the ingredients for life—exist in planetary systems beyond our own.
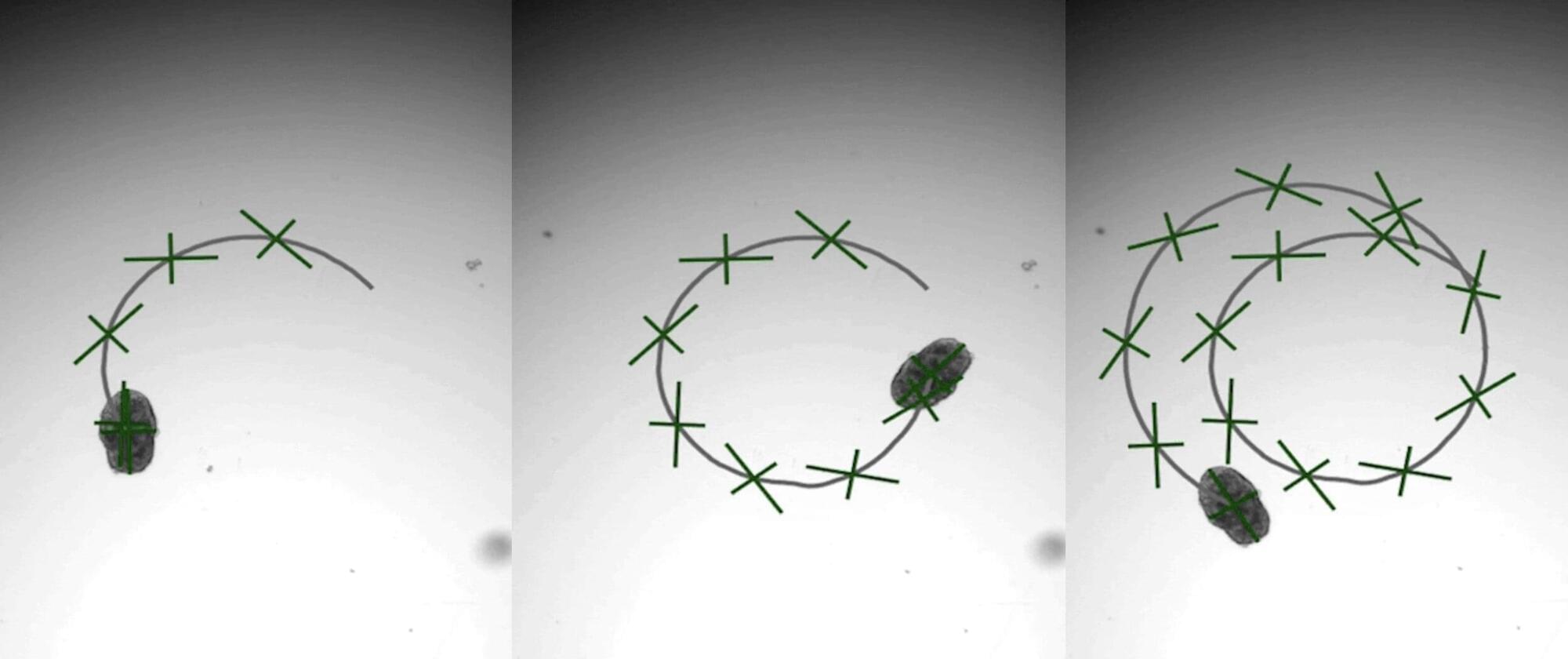
A brand-new engineering approach to generate “designer” biological robots using human lung cells is underway in Carnegie Mellon University’s Ren lab. Referred to as AggreBots, these microscale living robots may one day be able to traverse through the body’s complex environments to deliver desired therapeutic or mechanical interventions, once greater control is achieved over their motility patterns. In new research published in Science Advances, the group provides a novel tissue engineering platform capable of achieving customizable motility in AggreBots by actively controlling their structural parameters.
Biobots are microscopic, man-made biological machines capable of autonomous movement and programmability to perform specific tasks or behaviors. Previously, enabling biobots’ motility has been centered around using muscle fibers, which allow them to move by contracting and relaxing like real muscles.
A novel, alternative mechanism of actuation can be found by using cilia, the nanoscopic, hair-like, organic propellers that continuously move fluids in the body (like in the lungs) and help some aquatic creatures, like Paramecium or comb jellies, swim. However, a reliable way to control the exact shape and structure of a cilia-powered biobot (CiliaBot, for short), and thereby its motility outcome, has proven difficult to come by.
Shaping The Future Of Personalized Medicine — Professor Dragan[ ](https://www.facebook.com/PrimoracDragan?__cft__[0]=AZWpslTHjsy1a1kjedsti2RJw9yv6FhOXDFg2kyiufa2-D4Gk8TYoTy6HPaDPGARaq1EESF8mpBiV9Jjt2gpkh8Np3gpvzqTNu4cOTW-m31Hn4MVmEFyC6gnP5_-bMEdn1Gn81MUYh3llD5MqtPqF8dPWOZxq1Oo7MbC2g5664Of2FI4tc98YxJrFewUmig_tH0&__tn__=-]K-R)Primorac MD, PhD — Founder, St. Catherine Specialty Hospital
Professor Dragan Primorac, MD., PhD (https://www.draganprimorac.com/) is a globally recognized physician-scientist whose work spans personalized medicine, regenerative therapies, and forensic genetics.
From 2003 to 2009 Prof. Primorac served as the Minister of Science, Education and Sports of the Republic of Croatia. The Ministry of Science and Education of Croatia is the ministry in the Government of Croatia which is in charge of primary, secondary and tertiary education, research institutions and sports (https://mzom.gov.hr/en).
Prof. Primorac is the Founder of St. Catherine Specialty Hospital in Zagreb Croatia (https://www.stcatherine.com/), the official hospital of the Croatian Olympic Committee as well as the official hospital of the Croatian Football Federation. St. Catherine Hospital is affiliated with four medical schools and the Ministry of Science and Education recently announced that the St. Catherine Hospital became Scientific Center of Excellence for the Personalized Medicine\.
Using the effect it could even be possible to generate photons from the quantum vacuum.

Harnessing the power of AI, a research team at the MRC-University of Glasgow Center for Virus Research has launched Viro3D —the most comprehensive database of human and animal virus protein structure predictions in the world.
The free and searchable AI-powered database offers a completely new, in-depth perspective on viruses, allowing us to quickly learn more about their origins and evolution.
Although virus particles are the most abundant biological entities on our planet, these tiny structures remain among the least well-understood. Insights into the key protein structures within viruses have, until now, only been achieved through slow and laborious research work, a pace that has impacted our ability to develop treatments and vaccines at speed.