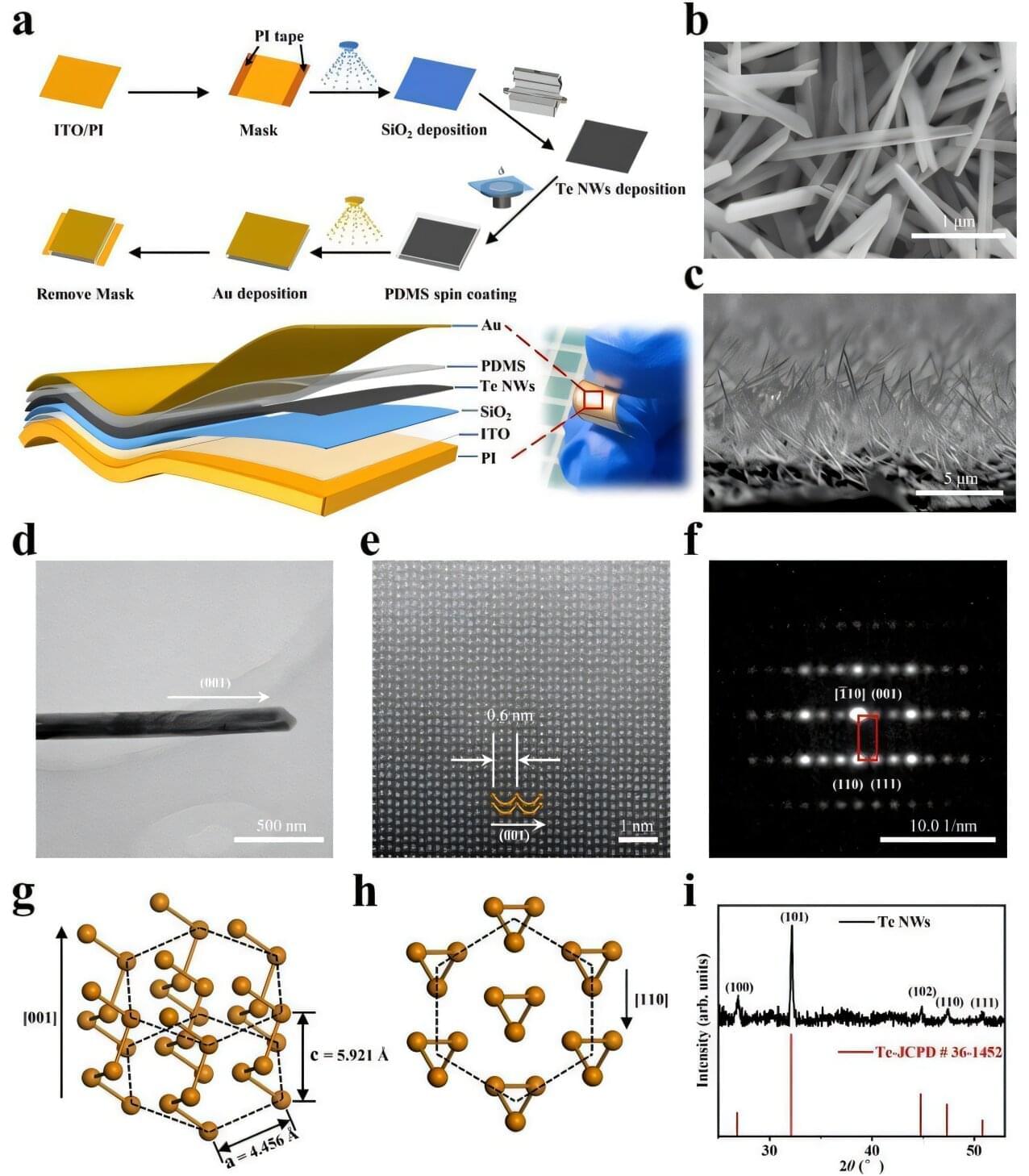
Cars from companies like Tesla already promise hands-free driving, but recent crashes show that today’s self-driving systems can still struggle in risky, fast-changing situations.
Now, researchers say the next safety upgrade may come from an unexpected source: The brains of the people riding inside those cars.
In a new study appearing in Cyborg and Bionic Systems, Chinese researchers tested whether monitoring passengers’ brain activity could help self-driving systems make safer decisions in risky situations.