Shai-Hulud v2 breached npm and Maven, impacting 28,000+ repos and leaking 11,858 secrets.


Why did all eight detection tools identically fail where the SOC succeeded?
What all these organizations have in common is a balanced investment across the alert lifecycle, which doesn’t neglect their SOC.
This article examines how investing in the SOC is indispensable for organizations that have already allocated significant resources to detection tools. Additionally, a balanced SOC investment is crucial for maximizing the value of their existing detection investments.

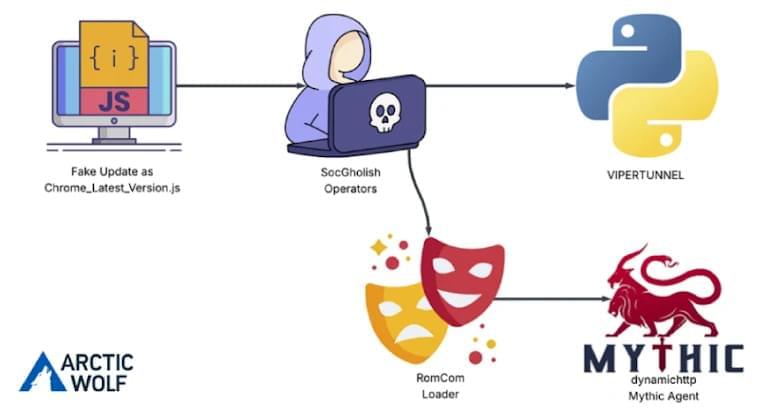

A vulnerability in the ‘node-forge’ package, a popular JavaScript cryptography library, could be exploited to bypass signature verifications by crafting data that appears valid.
The flaw is tracked as CVE-2025–12816 and received a high severity rating. It arises from the library’s ASN.1 validation mechanism, which allows malformed data to pass checks even when it is cryptographically invalid.
“An interpretation-conflict vulnerability in node-forge versions 1.3.1 and earlier enables unauthenticated attackers to craft ASN.1 structures to desynchronize schema validations, yielding a semantic divergence that may bypass downstream cryptographic verifications and security decisions,” reads the flaw’s description in the National Vulnerabilities Database (NVD).
For decades, we were taught the universe is 13.8 billion years old. Textbooks repeated it. Documentaries swore by it. Every model of cosmic history depended on it.
But now the numbers are breaking.
The James Webb Space Telescope is detecting galaxies that shouldn’t exist, stars older than our timeline allows, and cosmic structures so mature they overturn everything we thought we understood.
Reality is being rewritten in real time.
New evidence points to a universe that could be 26.7 billion years old – nearly double the age we believed. And if that’s true, then the biggest question becomes unavoidable.
What came before the Big Bang?
The answer is colder, emptier, and far stranger than anything in standard cosmology.
Get ready. We’re going back to the moment before everything.
COPYRIGHT DISCLAIMER:
All content in this video is original or sourced from licensed royalty-free libraries. Scientific material is used under fair use for educational analysis. The script, narration, music, SFX, and visuals are produced using legally obtained and properly credited resources.
Euclid’s first data release is here — and it’s already transforming our understanding of galaxy evolution, black hole growth, and the hidden structure of the Universe. From secondary galactic nuclei to rare ionized systems and newly revealed dwarf galaxies, Euclid is opening a new chapter in cosmology. To learn more about Euclid’s First Data Release, you can watch our full video on YouTube.
Paper link : https://arxiv.org/pdf/2503.
Chapters:
00:00 Introduction.
00:49 DISCOVERY
03:59 SCIENTIFIC IMPORTANCE & THEORIES
07:17 IMPLICATIONS & WHAT’S NEXT
10:49 Outro.
11:03 Enjoy.
MUSIC TITLE : Starlight Harmonies.
MUSIC LINK : https://pixabay.com/music/pulses-star… our website for up-to-the-minute updates: www.nasaspacenews.com Follow us Facebook: / nasaspacenews Twitter:
/ spacenewsnasa Join this channel to get access to these perks:
/ @nspacenews #NSN #NASA #Astronomy.
Visit our website for up-to-the-minute updates: