A typical human cell is metabolically active, roaring with chemical reactions that convert nutrients into energy and useful products that sustain life. These reactions also create reactive oxygen species, dangerous by-products like hydrogen peroxide which damage the building blocks of DNA in the same way oxygen and water corrode metal and form rust. Similar to how buildings collapse from the cumulative effect of rust, reactive oxygen species threaten a genome’s integrity.
Cells are thought to delicately balance their energy needs and avoid damaging DNA by containing metabolic activity outside the nucleus and within the cytoplasm and mitochondria. Antioxidant enzymes are deployed to mop up reactive oxygen species at their source before they reach DNA, a defensive strategy that protects the roughly 3 billion nucleotides from suffering potentially catastrophic mutations. If DNA damage occurs anyway, cells pause momentarily and carry out repairs, synthesizing new building blocks and filling in the gaps.
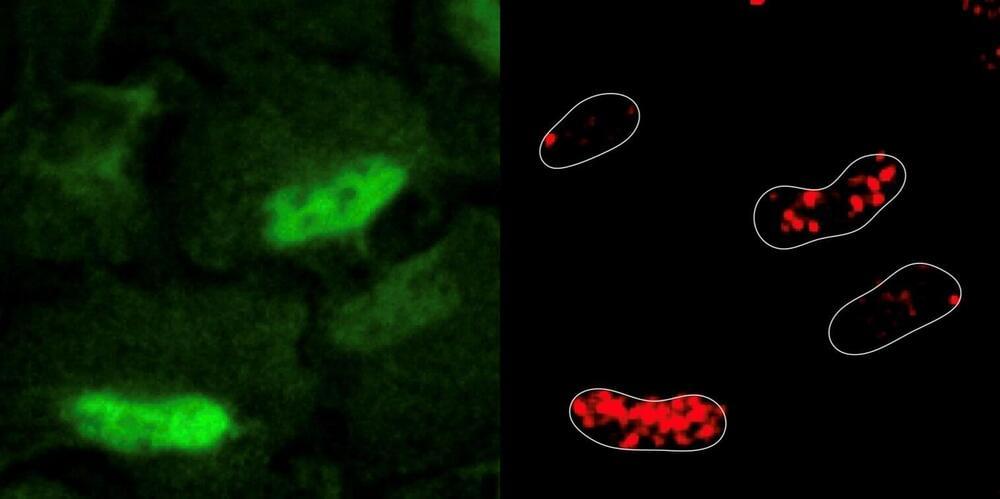
Despite the central role of cellular metabolism in maintaining genome integrity, there has been no systematic, unbiased study on how metabolic perturbations affect the DNA damage and repair process. This is particularly important for diseases like cancer, characterized by their ability to hijack metabolic processes for unfettered growth.