Imagine a skin cream that heals damage occurring throughout the day when your skin is exposed to sunlight or environmental toxins. That’s the potential of a synthetic, biomimetic melanin developed by scientists at Northwestern University.
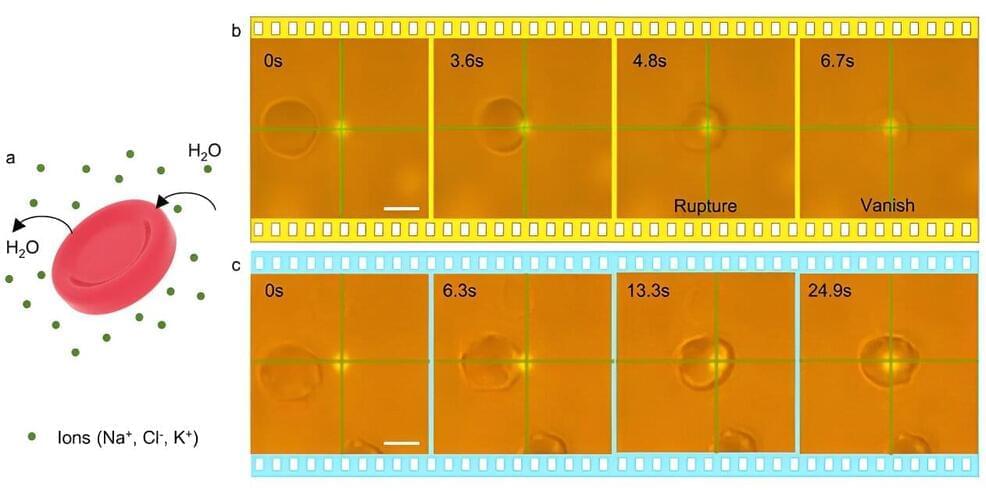
In a new study, the scientists show that their synthetic melanin, mimicking the natural melanin in human skin, can be applied topically to injured skin, where it accelerates wound healing. These effects occur both in the skin itself and systemically in the body.
When applied in a cream, the synthetic melanin can protect skin from sun exposure and heals skin injured by sun damage or chemical burns, the scientists said. The technology works by scavenging free radicals, which are produced by injured skin such as a sunburn. Left unchecked, free radical activity damages cells and ultimately may result in skin aging and skin cancer.


 עברית (Hebrew)
עברית (Hebrew)