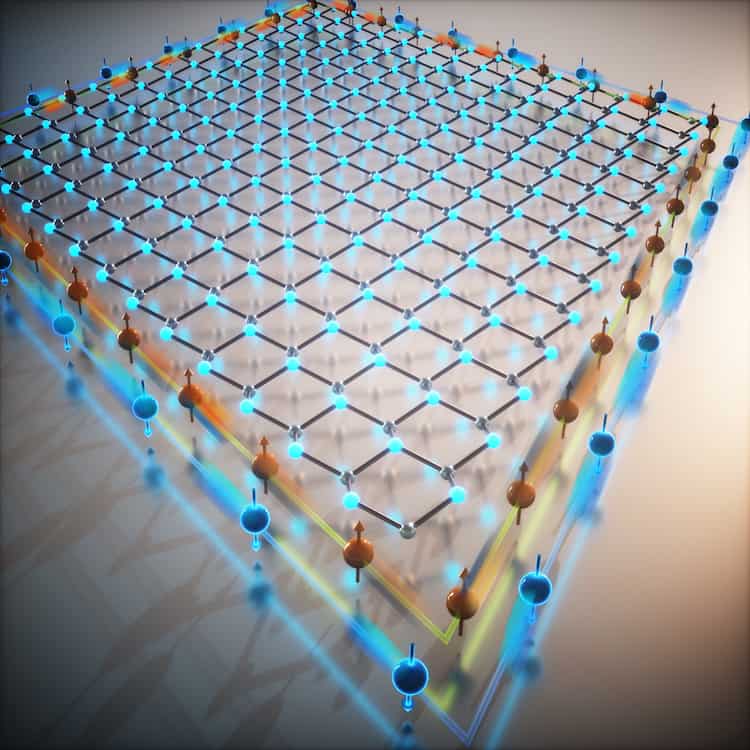
Ultra-high pressure can have strange effects in physics and chemistry, and in a new study, high-pressure modeling has led to the prediction of four new compounds: compounds that don’t form in normal ways, have crystal structures we’ve never seen before, and can even act as superconductors in certain temperatures.
Those compounds are Li14 Cs, Li8Cs, Li7Cs, and Li6Cs, and they’re all formed from lithium (Li) and cesium (Cs) – though not in a conventional way. All four are superconductors, which means electricity can flow through them without resistance or energy loss.
The scientists behind the study used a special crystal structure prediction algorithm called USPEX (Universal Structure Predictor: Evolutionary Xtallography) to find these new compounds. It’s known as an evolutionary algorithm, using a range of methods to figure out the probability of how atoms will link together.