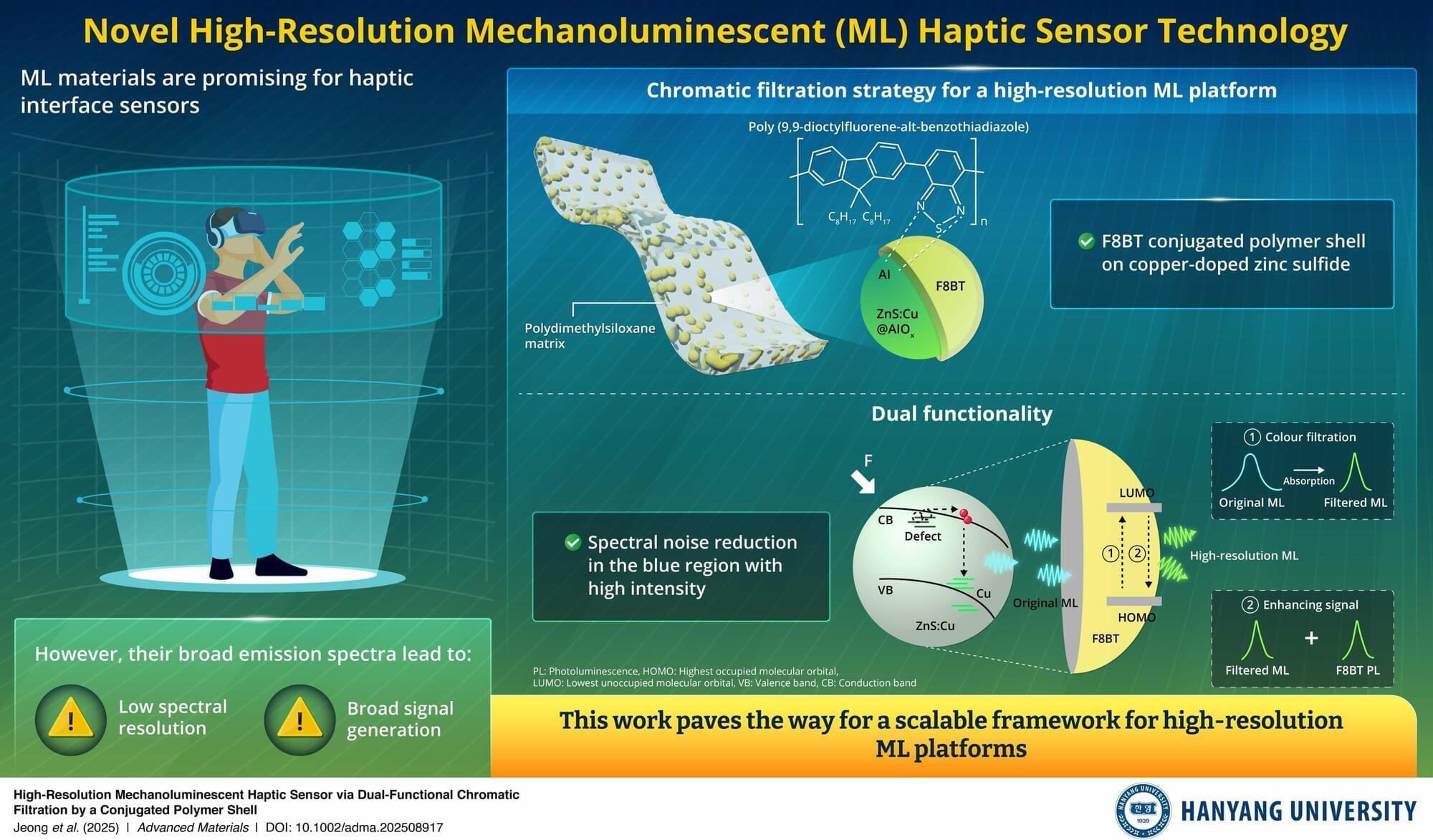
Mechanoluminescent (ML) materials are attractive for haptic interface sensors for next-generation technologies, including bite-controlled user interface, health care motion monitoring, and piconewton sensing, because they emit light under mechanical stimulation without an external power source. However, their intrinsically broad emission spectra can degrade resolution and introduce noise in sensing applications, necessitating further technological development.
Addressing this knowledge gap, a team of researchers from the Republic of Korea and the UK, led by Hyosung Choi, a Professor at the Department of Chemistry at Hanyang University, and including Nam Woo Kim, a master’s student at Hanyang University, recently employed a chromatic filtration strategy to pave the way to high-resolution ML haptic sensors. Their findings are published in the journal Advanced Materials.
In this study, the team coated the conjugated polymer poly(9,9-dioctylfluorene-alt-benzothiadiazole) (F8BT) onto ZnS: Cu to selectively suppress emission below 490 nm, narrowing the full width at half maximum from 94 nm to 55 nm.