UpCrypter phishing since Aug 2025 uses fake voicemails, RAT payloads, and anti-analysis, hitting global industries.





Scientists at the Medical Research Council’s Laboratory of Molecular Biology say they’ve engineered a bacteria whose genetic code is more efficient than any other lifeform on Earth.
They call their creation “Syn57,” a bioengineered strain of E. coli — yes, the same bad boy that can make you extremely sick if you eat an undercooked hot dog — which uses seven less codons than all life on earth. A codon, put simply, is a three-letter sequence found in DNA and RNA which delivers instructions for amino acids, a fundamental “building block” of life.
For the past billions years or so, all known life on earth has used 64 codons. Scientists cracked the code detailing which codons corresponded to which amino acids — mapping the standard genetic code, in other words — in 1966, revealing only 20 total amino acids.


The development of highly complex chemical systems, self-assembled by the donor-acceptor and/or noncovalent interactions, lies at the core of supramolecular chemistry.
Recently, increasing attention has been paid to structurally adaptable molecular systems and robust noncovalent microporous materials (NPMs), also known as molecular porous materials (MPMs) or porous molecular crystals (PMCs), based on the self-assembly of discrete molecules driven by weak interactions. The utilization of molecular metal clusters as building units of NPMs is a promising strategy, combining the versatile functionality of organic and inorganic subunits with the softness and flexibility of molecular solids controlled by noncovalent interactions.
However, the development of robust porous functional frameworks based on self-assembly driven by noncovalent forces is still highly challenging.
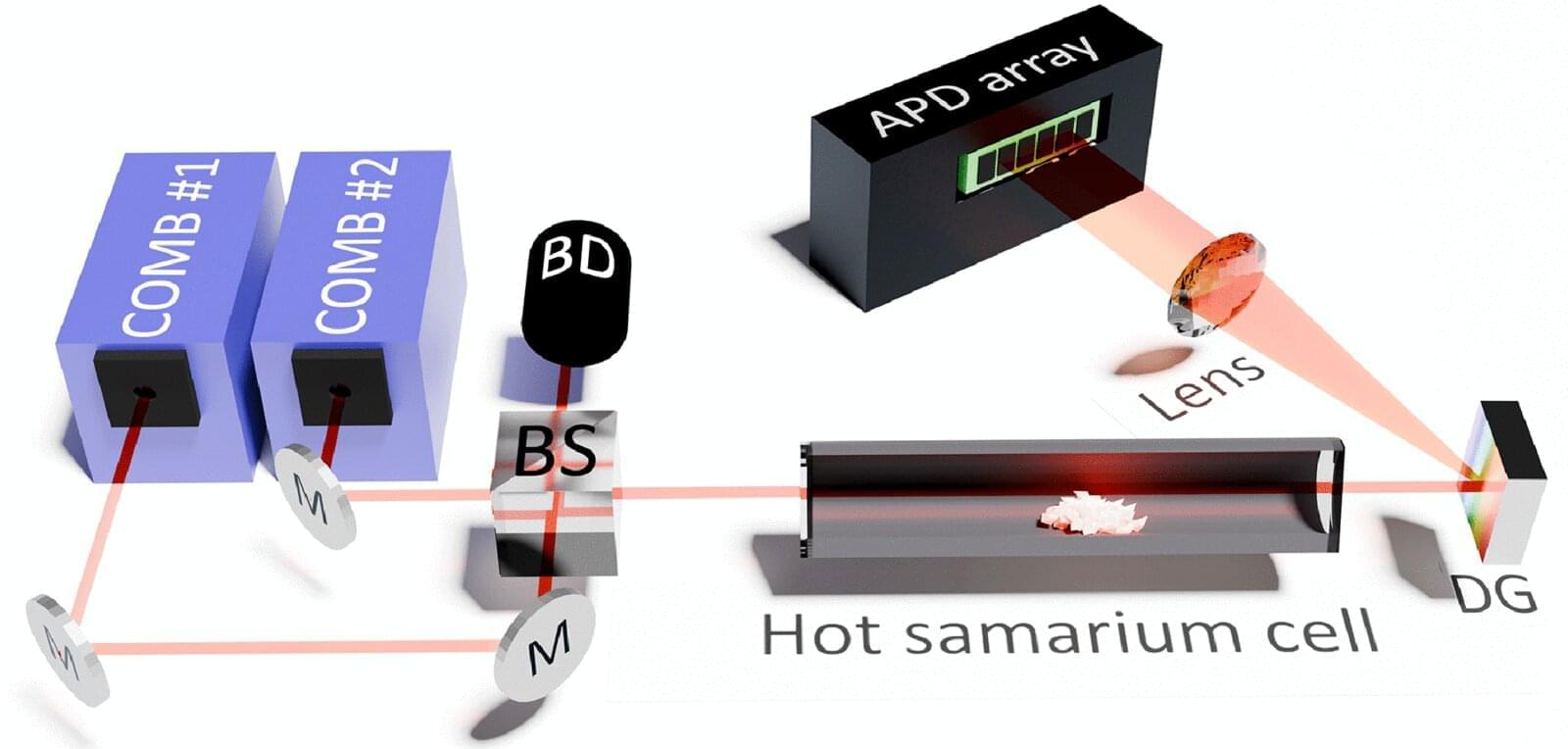
Researchers at Johannes Gutenberg University Mainz (JGU) and the Helmholtz Institute Mainz (HIM) have developed a novel method for investigating the internal structure of atoms and discovered previously unknown atomic transitions in samarium, a rare earth element. Their findings were published in the journal Physical Review Applied.
The ability to describe the internal structure of atoms is important not only for understanding the composition of matter, but also for designing new experiments to explore fundamental physics. Specific experiments require samples of atoms or molecules with particular properties, which depend heavily on the phenomenon to be explored. However, the knowledge of the energy-level structure of many atoms remains incomplete, particularly in the case of the rare earth and actinide atoms.
Spectroscopy is one of the most widely used techniques for studying the structure of atoms. This technique is based on the principle that electrons absorb or emit energy when they move between energy levels in an atom. Each element has a unique set of wavelengths of light that are emitted or absorbed due to these transitions. This is known as the atomic spectrum.
