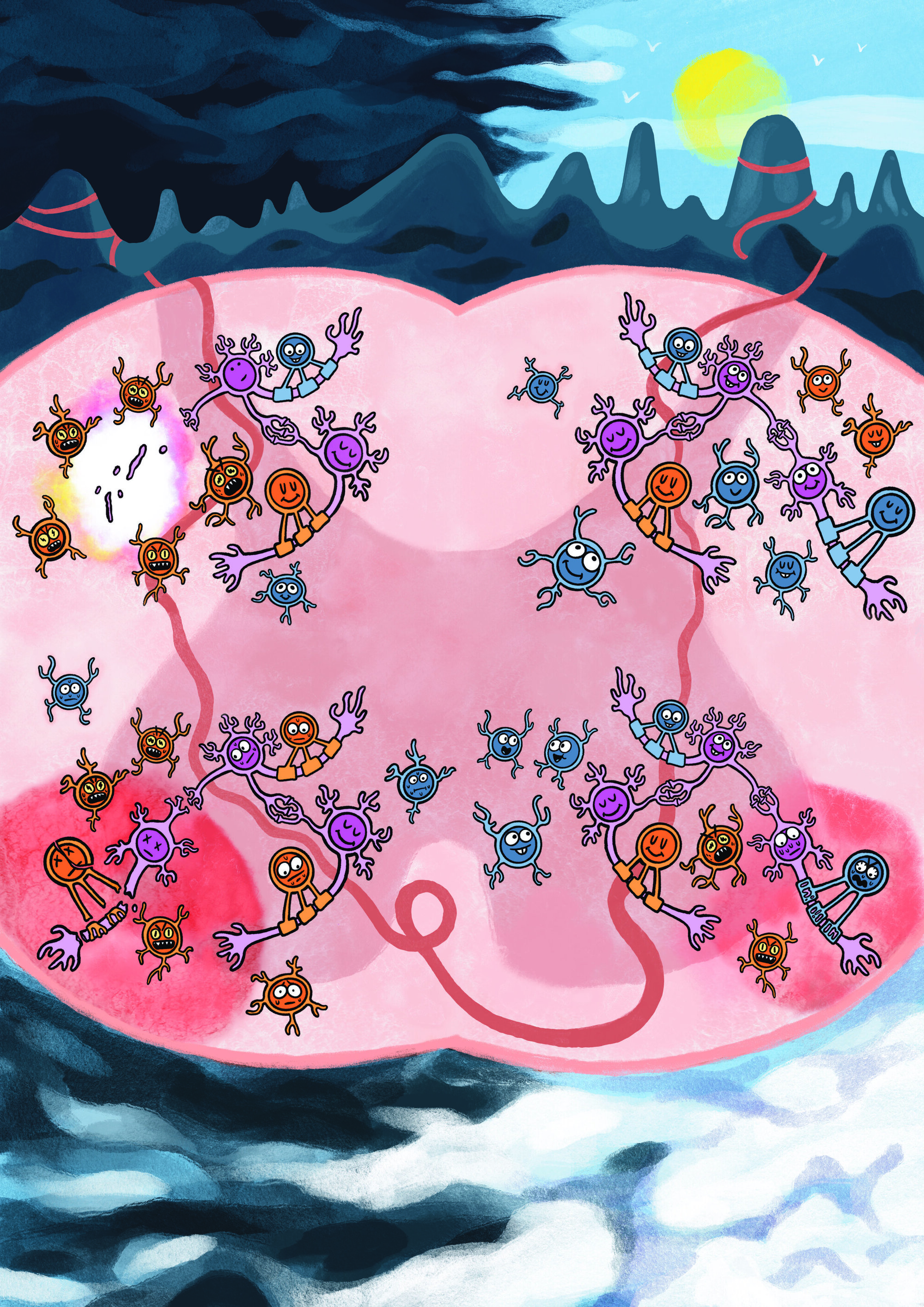
Multiple sclerosis (MS) is a chronic autoimmune disease characterized by the disruption of nerve signals and various associated neurological symptoms, ranging from vision problems to numbness, weakness, fatigue and cognitive impairments. These symptoms emerge when the immune system starts to attack mature oligodendrocytes (MOLs), specialized cells that produce the protective sheath surrounding nerve fibers (i.e., myelin).
There are several subtypes of MOLs, which might exhibit different immune cell-like genetic responses in patients diagnosed with MS. While various studies have investigated the neural and molecular underpinnings of MS, how these different cell subtypes respond as the disease progresses has not yet been elucidated.
Researchers at Karolinska Institute in Sweden recently carried out a mouse study aimed at mapping how different MOL subtypes might differ in their sensitivity to neuroinflammation across different stages of MS.