Powered by OpenAI technology, the A.I. version of a popular Snapchat influencer is made over $70,000 in a week, marking the beginning of an ethically-thorny era.
As the world strives to ensure everyone has access to their fundamental human rights, technology is a crucial innovator for making lives better.
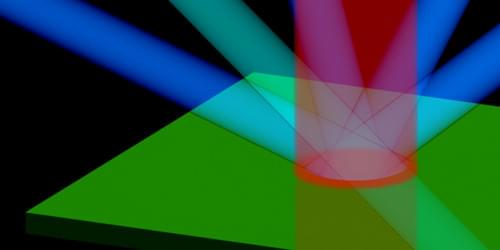
Floating 3D Display Clock
Posted in futurism
Moritz v. Sivers’ clock uses a beamsplitter and retroreflective foil to make digits float and rotate in midair.
Dr_Microbe/iStock.
To address these concerns, scientists at SNIPR BIOME company have been working on developing a targeted approach to kill harmful bacteria while saving the essential ones precisely.
Understanding the possible quantum-driven behaviors of biological systems could aid in treating injuries or in developing cures for diseases, but research in the field has been pushed to the sidelines. It’s time for that to change.
A study of the mechanical forces in certain immune cells may give new insights into how organisms deal with ever-evolving pathogens.
To fight disease, many organisms have an adaptive immune system, which learns the molecular shapes of foreign elements (antigens) and remembers them to mount a defense against future infections. In vertebrates, the learning stage involves a remarkable cycle of evolution within an individual animal—a cycle called affinity maturation, which involves a type of immune cell called a B cell (Fig. 1). In this process, B cells are selected to have receptors that bind strongly to specific antigens. However, if these cells become too specialized, they risk becoming unresponsive to slightly mutated pathogens. Fortunately, the immune system can limit affinity maturation to retain a range of specificities for target pathogens. Just how the immune system is able to do that is the subject of a fascinating new study by Hongda Jiang and Shenshen Wang from the University of California, Los Angeles [1].
A novel laser cooling and trapping technique squeezes large numbers of molecules into a confined space while keeping them ultracold.
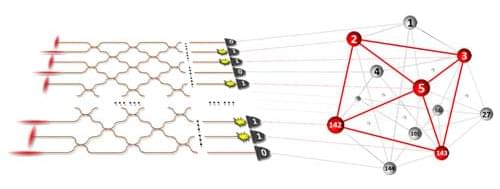
A quantum photonic device can perform some real-world tasks more efficiently than classical computers.
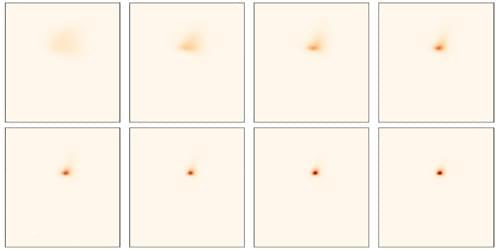
Experiments probing quasiparticles in semiconductor microcavities offer unprecedented insights into the dynamics of quantum fluids of light.
Superfluidity [1, 2], the ability of a fluid to flow without friction, isn’t restricted to systems described by hydrodynamics. Over a decade ago, optics researchers started to take an interest in superfluids and other quantum fluids [3], driven by the realization that light propagating in a nonlinear medium can exhibit quantum hydrodynamics features [4]. Two platforms emerged for the study of these “fluids of light”: semiconductor microcavities in which photons are confined [5] and propagating geometries in which photons travel in a bulk medium [6–8]. Both configurations allow photons to acquire an effective mass and experience an effective mutual interaction—properties that can lead them to collectively behave as a quantum fluid.