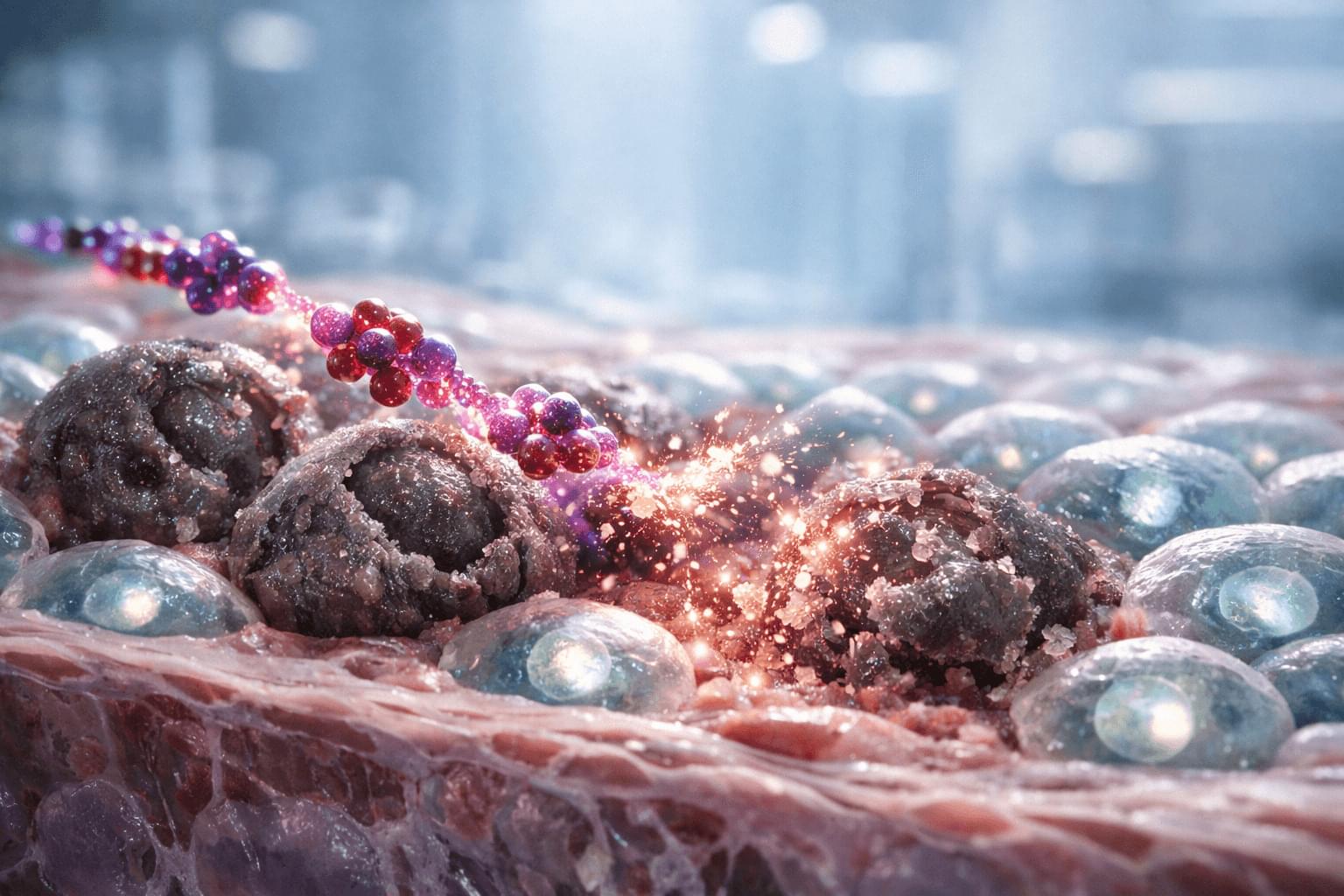
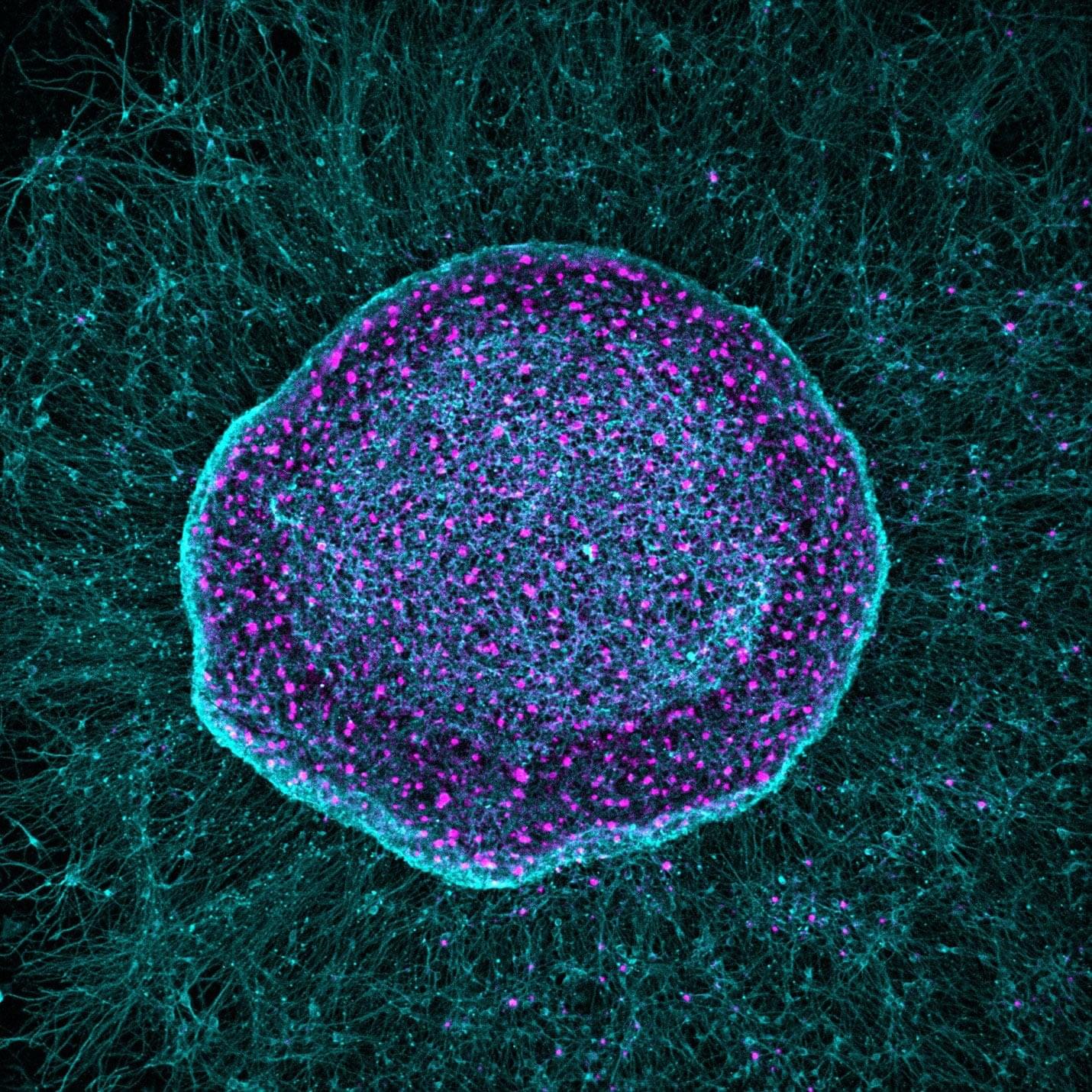
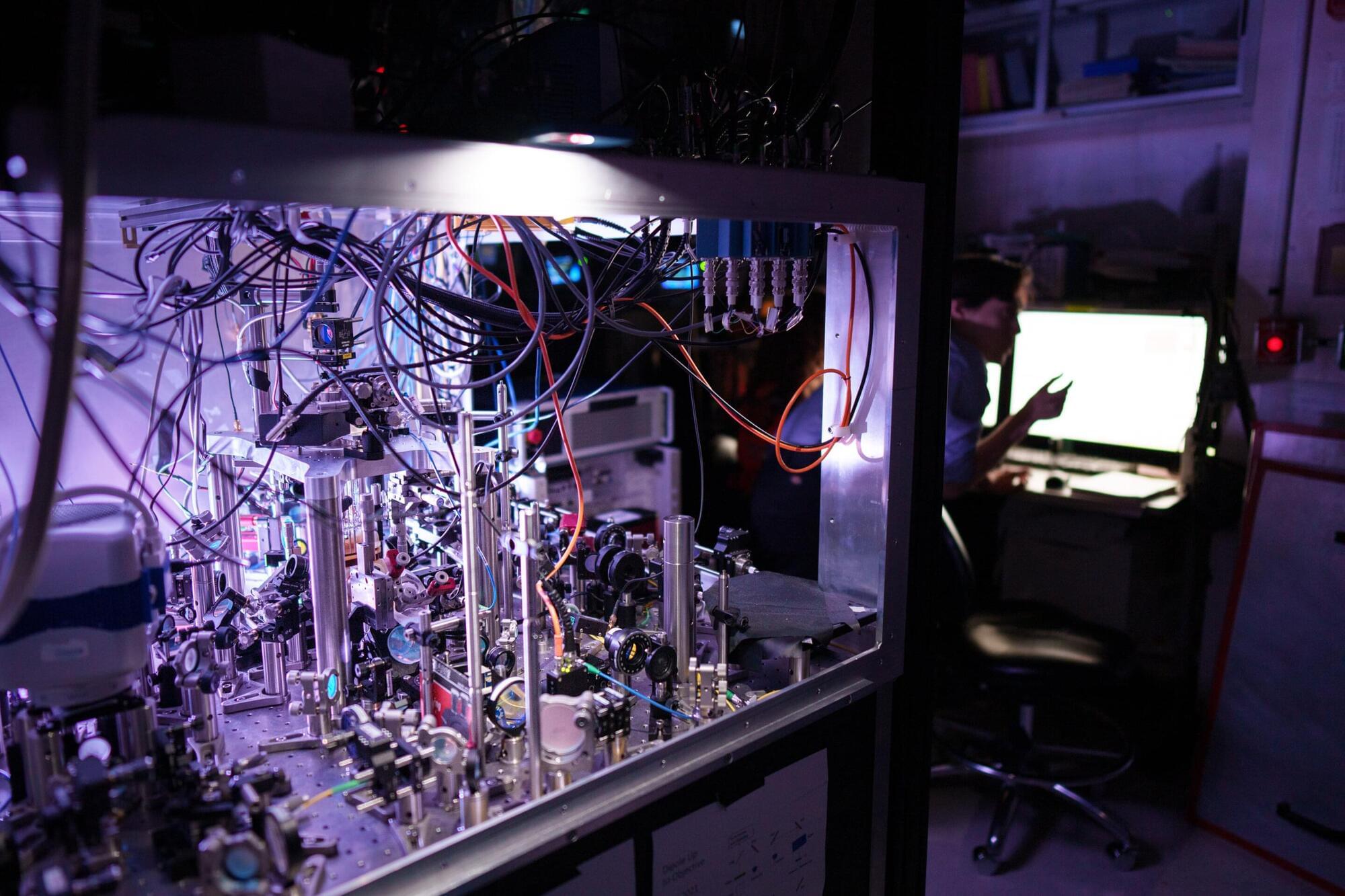
An Australian startup has unveiled the world’s first commercial biological computer (CL1), running on living human cells. CL1 is claimed to be capable of learning and adapting faster than standard silicon-based AI.
#News #ai #technology #futureofhealth #computer #braincells #Reuters #Newsfeed.
Read the story here:
👉 Subscribe: http://smarturl.it/reuterssubscribe.
Keep up with the latest news from around the world: https://www.reuters.com/
Follow Reuters on Facebook: / reuters.
Follow Reuters on Twitter: / reuters.
Follow Reuters on Instagram: https://www.instagram.com/reuters/?hl=en