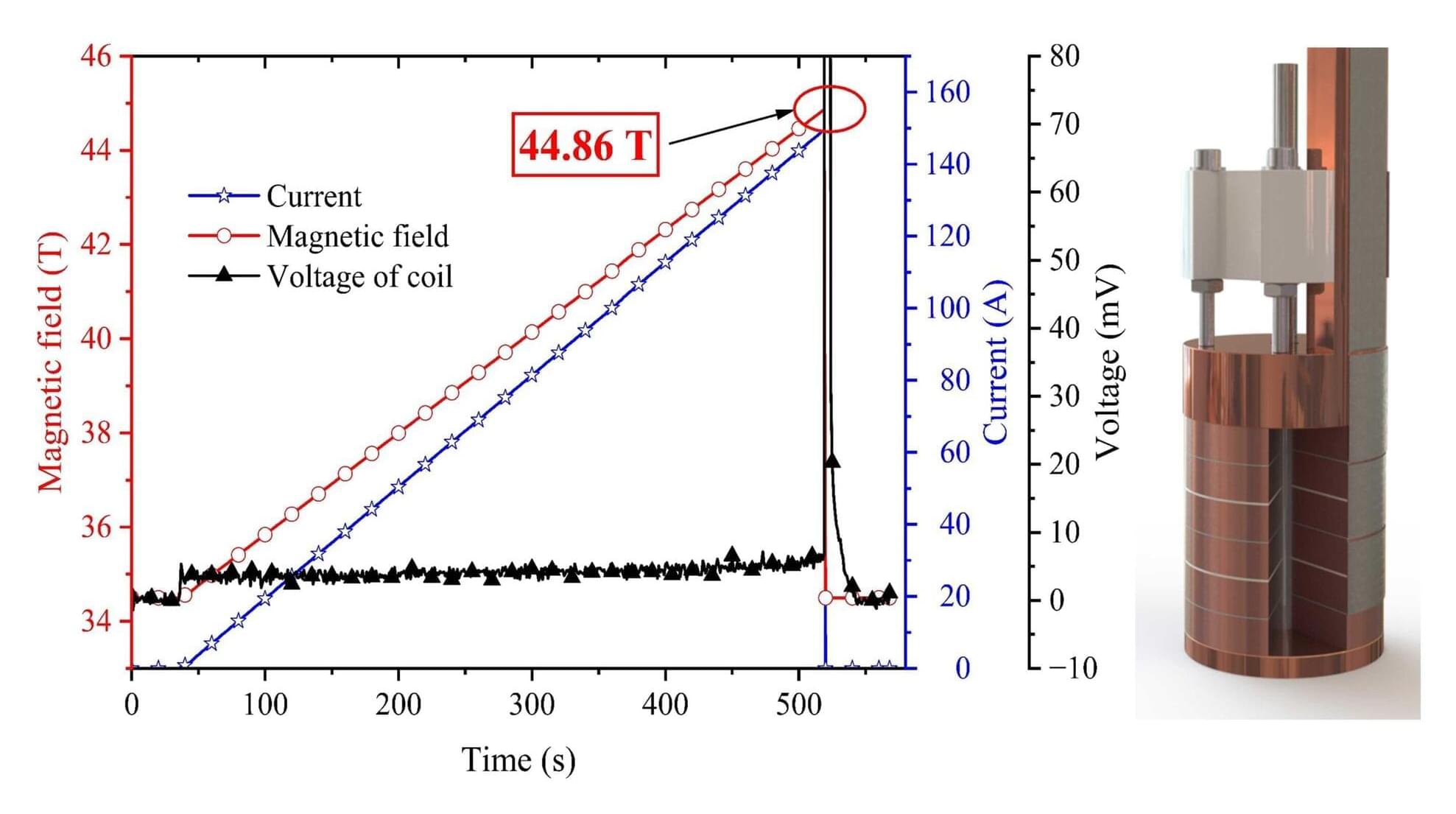
When it comes to understanding the universe, what we know is only a sliver of the whole picture.
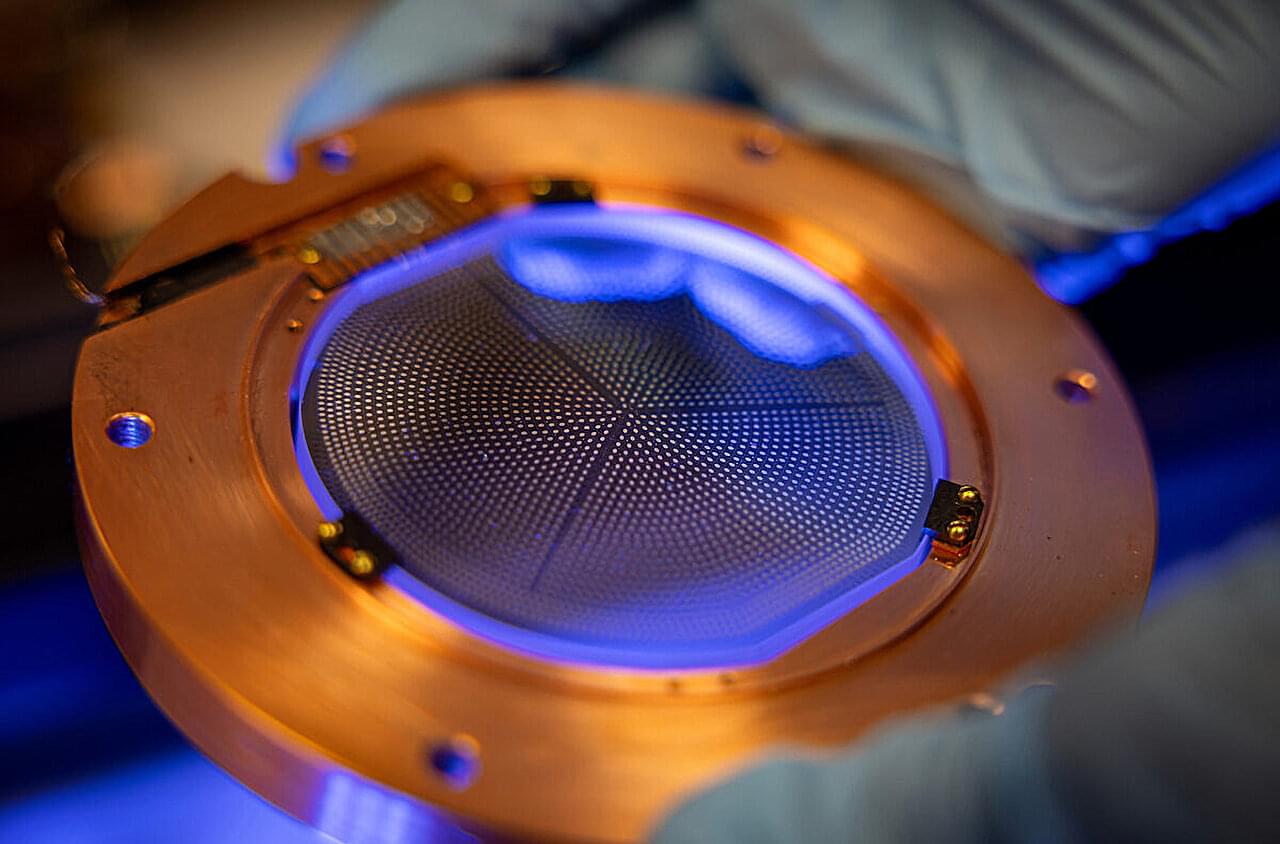
Dark matter and dark energy make up about 95% of the universe, leaving only 5% “ordinary matter,” or what we can see. Dr. Rupak Mahapatra, an experimental particle physicist at Texas A&M University, designs highly advanced semiconductor detectors with cryogenic quantum sensors, powering experiments worldwide and pushing the boundaries to explore this most profound mystery.
Mahapatra likens our understanding of the universe—or lack thereof—to an old parable: “It’s like trying to describe an elephant by only touching its tail. We sense something massive and complex, but we’re only grasping a tiny part of it.”