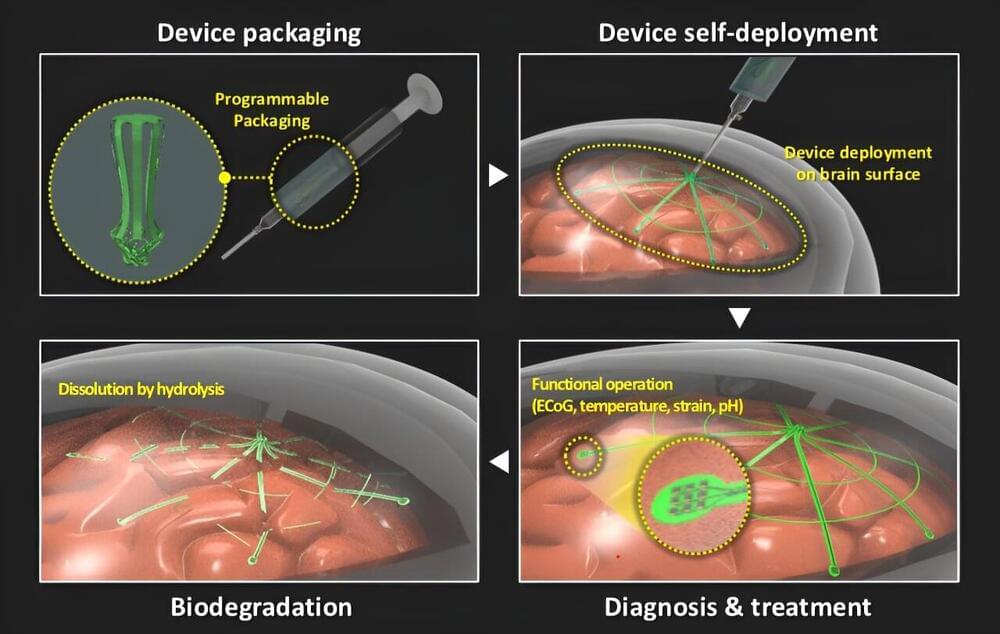
Brain-machine interfaces (BMIs) have emerged as a promising solution for restoring communication and control to individuals with severe motor impairments. Traditionally, these systems have been bulky, power-intensive, and limited in their practical applications. Researchers at EPFL have developed the first high-performance, Miniaturized Brain-Machine Interface (MiBMI), offering an extremely small, low-power, highly accurate, and versatile solution.
Published in the latest issue of the IEEE Journal of Solid-State Circuits (“MiBMI: A 192/512-Channel 2.46mm 2 Miniaturized Brain-Machine Interface Chipset Enabling 31-Class Brain-to-Text Conversion Through Distinctive Neural Codes”) and presented at the International Solid-State Circuits Conference, the MiBMI not only enhances the efficiency and scalability of brain-machine interfaces but also paves the way for practical, fully implantable devices. This technology holds the potential to significantly improve the quality of life for patients with conditions such as amyotrophic lateral sclerosis (ALS) and spinal cord injuries.
An image of the chip. (Image: EPFL)