In a high-security Shenzhen laboratory, Chinese scientists have built a prototype of a machine capable of producing cutting-edge semiconductor chips that power artificial intelligence, smartphones and weapons central to Western military dominance.


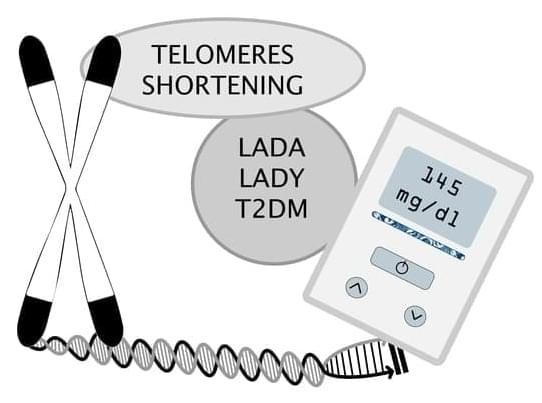
This study aimed to explore the role of telomere length in three different diabetes types: latent autoimmune diabetes of adulthood (LADA), latent autoimmune diabetes in the young (LADY), and type 2 diabetes mellitus (T2DM). A total of 115 patients were included, 72 (62.61%) had LADA, 30 (26.09%) had T2DM, and 13 (11.30%) had LADY. Telomere length was measured using real-time Polymerase Chain Reaction. For statistical analysis, we used the ANOVA test, X2 test, and the Mann–Whitney U test. Patients with T2DM had higher BMI compared to LADA and LADY groups, with a BMI average of 31.32 kg/m2 (p = 0.0235). While the LADA group had more patients with comorbidities, there was not a statistically significant difference (p = 0.3164, p = 0.3315, p = 0.3742 for each of the previously mentioned conditions).

Money is supposed to be the reward for effort. Elon Musk thinks it eventually becomes unnecessary paperwork.
While talking with Indian entrepreneur and investor Nikhil Kamath on the “People by WTF” podcast last month, the Tesla and SpaceX CEO and richest man in the world returned to a theme he’s raised before. It’s one he treats less like a theory and more like an inevitability. As artificial intelligence and robotics accelerate, Musk believes society moves past jobs, past income debates, and straight into something stranger.
Prediction Market powered by.
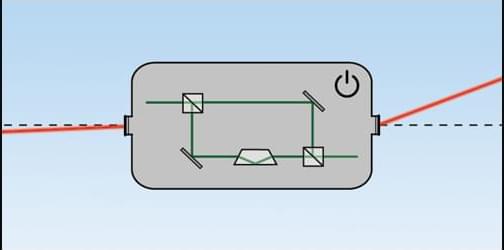
Measuring very small displacements of a laser beam is important in many areas of science and technology, such as in an atomic force microscope. A quantum trick called weak-value amplification (WVA) has previously led to extremely sensitive measurements of beam shifts within interferometers. Now Carlotta Versmold of the Ludwig Maximilian University of Munich and her colleagues have extended such measurements to beam displacements outside of an interferometer [1]. For example, a laser beam reflecting off of a distant window could encode vibrations resulting from conversations inside the building.
In the WVA version applicable to shifts within an interferometer, a light beam is split and routed along two slightly unequal paths that later merge and lead to two output ports—a “bright” port where the beams largely reinforce one another and a “dark” port where they mostly cancel each other out. Any slight displacement of either beam is amplified in the position of the dim spot at the dark port. However, shifts in the beam entering the interferometer lead to offsetting shifts of the internal beams and thus to no measurable signal.
To extend the method to shifts of the incoming beam, Versmold and her colleagues added a so-called Dove prism to one of the beam paths. This type of prism generates an additional reflection, which effectively leads to opposite shifts in the two paths, resulting in an amplified signal at the dark port.

A new artificial neural-network architecture opens a window into the workings of a tool previously regarded as a black box.
Thanks to the extremely large datasets and computing power that have become available in recent years, a new paradigm in scientific discovery has emerged. This new approach is purely data driven, using large amounts of data to train machine-learning models―typically neural networks―to predict the behavior of the natural world [1]. The most prominent achievement of this new methodology has arguably been the AlphaFold model for predicting protein folding (see Research News: Chemistry Nobel Awarded for an AI System That Predicts Protein Structures) [2]. But despite such successes, these data-driven approaches suffer a major drawback in that they are generally “black boxes” that offer no human-accessible understanding of how they make their predictions. This shortcoming also extends to the models’ inputs: It is often desirable to build known domain knowledge into these models, but the data-driven approach excludes that option.

In an unprecedented celestial event, NASA’s Hubble Space Telescope (HST) captured the dramatic aftermath of colliding space rocks within a nearby planetary system.
When astronomers initially spotted a bright object in the sky, they assumed it was a dust-covered exoplanet, reflecting starlight. But when the “exoplanet” disappeared and a new bright object appeared, the international team of astrophysicists—including Northwestern University’s Jason Wang—realized these were not planets at all. Instead, they were the illuminated remains of a cosmic fender bender.
Two distinct, violent collisions generated two luminous clouds of debris in the same planetary system. The discovery offers a unique real-time glimpse into the mechanisms of planet formation and the composition of materials that coalesce to form new worlds.

With many people now heavily relying on electronic devices to communicate with others, connecting on a deeper level with others, particularly face-to-face, can prove challenging. Recent nationwide surveys and psychological studies suggest that today many people feel lonely, socially isolated and/or disconnected from others living in their same geographical area.
Understanding the factors that contribute to social connection could inform the development of more effective interventions aimed at reducing loneliness and improving people’s mental health or overall well-being. As communication is generally crucial for the formation of social bonds, listening behaviors and an openness towards what others share might be key drivers of social connection.
Researchers at University of North Carolina at Chapel Hill recently carried out a study aimed at testing this hypothesis by examining the behavior of strangers engaged in conversation with each other. Their findings, published in Communications Psychology, suggest that people who engage in high-quality listening behaviors tend to feel more socially connected to others, even if they are meeting them for the first time.

Traditional research into giftedness and expertise assumes that the key factors to develop outstanding achievements are early performance (e.g., in a school subject, sport, or in concerts) and corresponding abilities (e.g., intelligence, motor skills, musicality) along with many years of intensive training in a discipline. Accordingly, talent programs typically aim to select the top-performing youth and then seek to further accelerate their performance through intensive discipline-specific training.
However, this is apparently not the ideal way to promote young talent, as a team led by Arne Güllich, professor of sports science at RPTU University Kaiserslautern-Landau, has recently discovered.
The work is published in the journal Science.