The qubits are similar enough to those used by the likes of Google and IBM that they could slot into existing processors in the future.




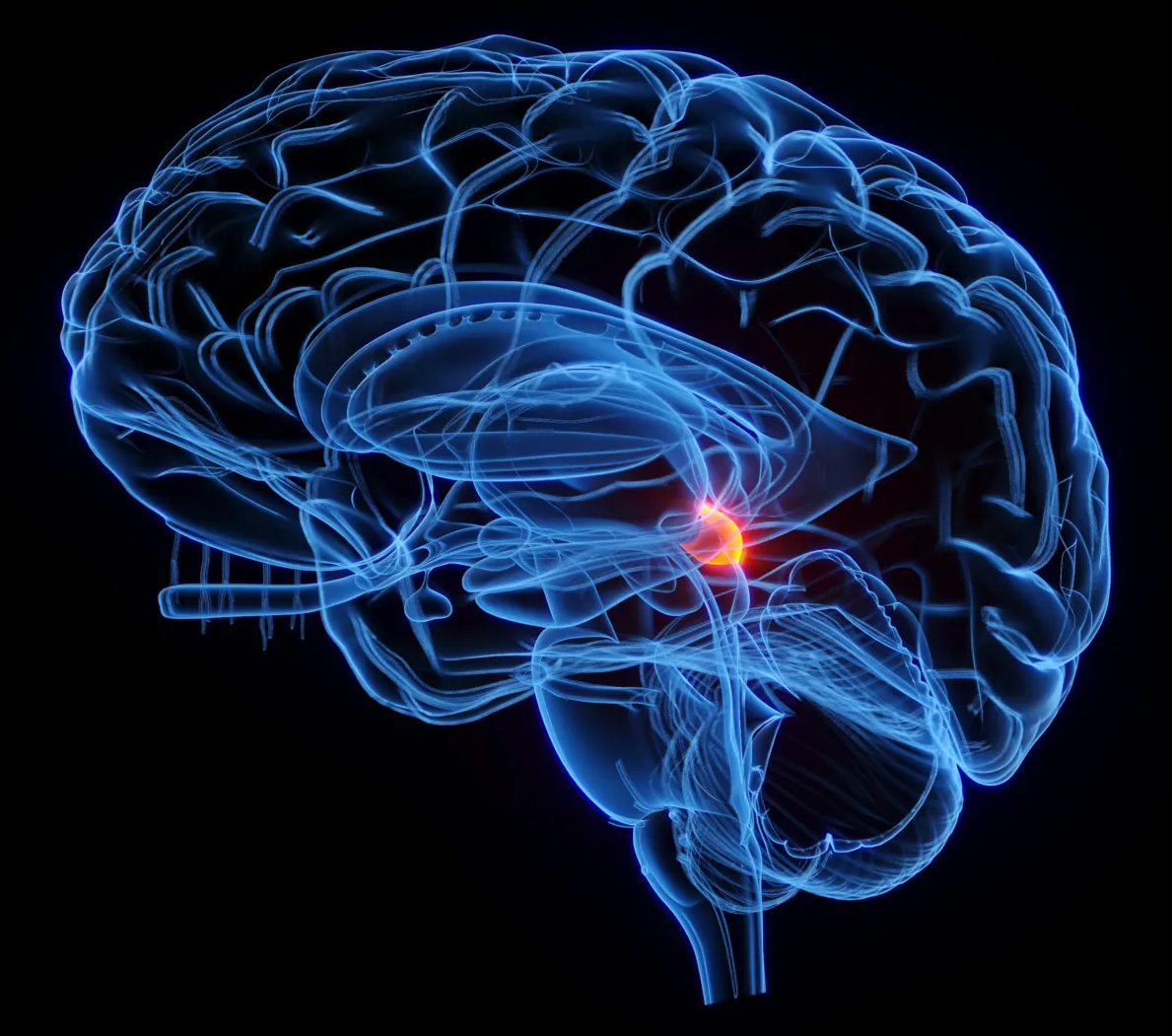
New research shows that the superior colliculus, a primitive brain region, can independently interpret visual information. This challenges long-held beliefs that only the cortex handles such complex computations. The discovery highlights how ancient neural circuits guide attention and perception, shaping how we react to the world around us.

Graphene can be made from plants and even carbon dioxide aswell as any other carbon based materials. This could make a near unlimited supply of graphene.
Muhammad taqi-uddeen safian, umirah syafiqah haron, and mohamad nasir mohamad ibrahim
Biomass waste has become a new source for producing graphene due to its carbon-rich structure and renewable nature. In this paper, the research on the conversion of bio-based graphene from different biomass wastes is summarised and discussed. This paper reviews the methods for converting biomass to bio-based graphene. There are two approaches for thermal degradation of biomass: thermal exfoliation and carbon growth. The purpose of the thermal treatment is to increase the carbon content by removing volatile matter from the biomass polymer chain. Pre-treatments that help to break down the complex structure of the biomass are discussed; pre-treatments also remove impurities from the said biomass. Lastly, the characteristics of bio-based graphene produced from different biomass and thermal treatments are summarised.

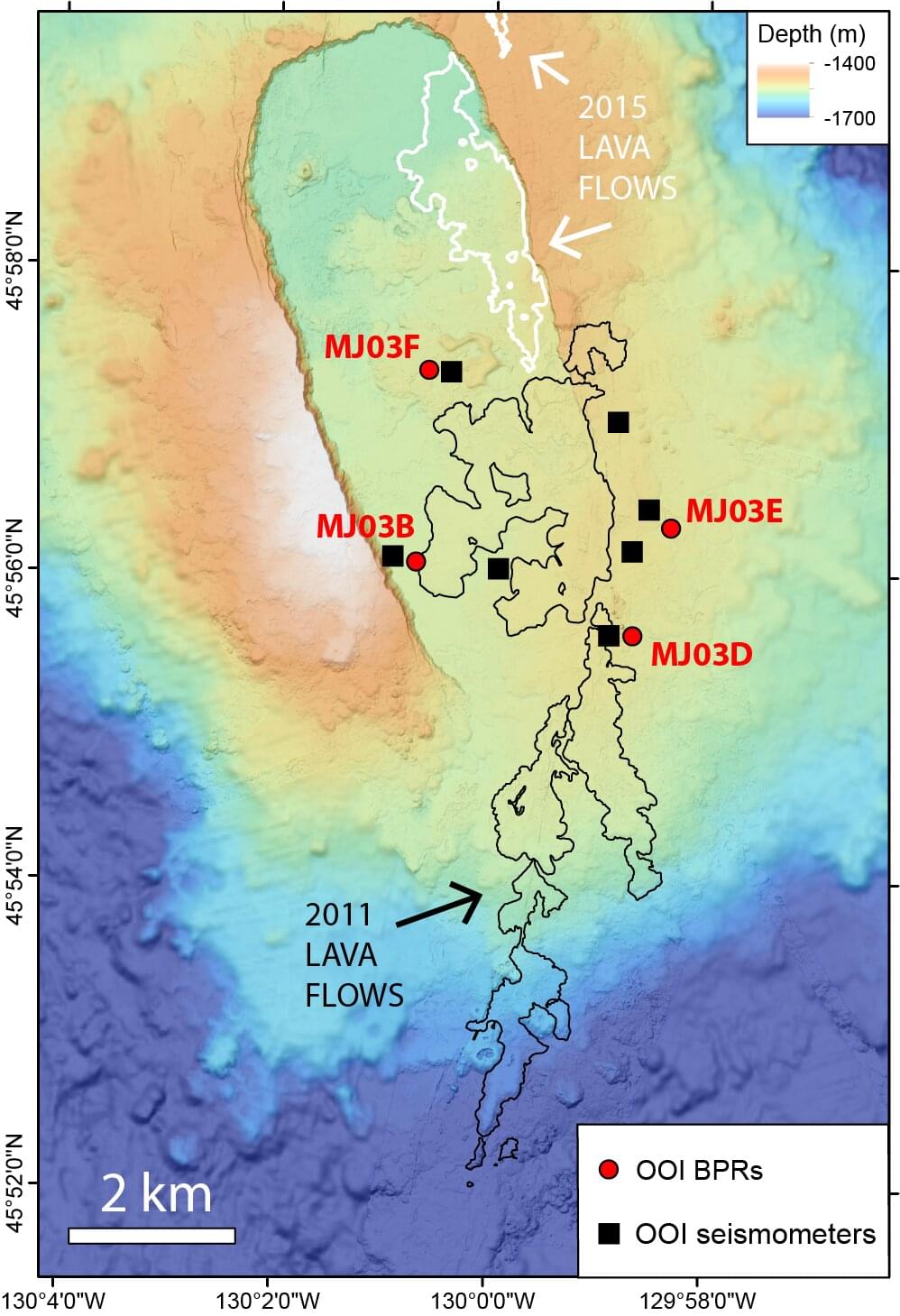
Currently, scientists struggle to forecast volcano eruption events, as no universally reliable, real-time eruption forecasting framework is available. Instead, researchers often rely on retrospective analysis to evaluate eruptions. And although much has been learned from doing this, it can sometimes introduce biases, such as data snooping, hindsight reinterpretation, and post-eruption model adjustment.
As a potential remedy to this problem, a group of researchers working with the Geohazards Crisis Observatory have launched an ongoing experiment focused on developing a physics-based eruption forecasting framework. The findings are published on the arXiv preprint server.
Robots aren’t always the most delicate of machines when handling fragile objects. They don’t have the lightness of touch of humans. But that could be about to change thanks to a new development in smart materials.
Researchers have developed a method for weaving flexible fibers that can be controlled by magnetic fields. Not only can this be used for robot hands to pick up objects like soft fruits, potato chips and worms, but it can also be used in a range of other applications. These include gloves that provide a realistic touch in virtual reality and breathable fabrics.
Blue Origin’s is launching their second New Glenn rocket, for mission NG-2, from Launch Complex 36 at Cape Canaveral Space Force Station. It will deploy NASA’s ESCAPADE twin spacecraft to study Mars’ magnetosphere and solar wind interactions, alongside a Viasat communications technology demonstration. Blue Origin is planning to propulsively land the booster down range on their droneship, Jacklyn.
Want to support what I do? Consider becoming a Patreon supporter for access to exclusive livestreams, our discord channel! — / everydayastronaut.
Or become a YouTube member for some bonus perks as well! — / @everydayastronaut.
The best place for all your space merch needs!
https://everydayastronaut.com/shop/
All music is original! Check it out anywhere you listen to music (Spotify, iTunes, Google Play, Amazon, etc) by searching Everyday Astronaut.
