Canadian Space Agency astronaut Jeremy Hansen will make history as the first Canadian to go on a mission around the Moon.



face_with_colon_three Year 1998
In ancient Greece, immortality was the province of gods who spun the length of each lifetime. The myth has a kernel of truth, because the ends of chromosomes are protected by specialized stretches of DNA called telomeres. Once these are snipped too much by imperfect copying, a cell goes into senescence and stops dividing. Now two reports show that, with the help of an enzyme called telomerase, human cells can divide forever in the laboratory without turning cancerous. The findings, reported in the January issue of Nature Genetics, could ease the way to new treatments for burn victims, diabetics, and patients with other diseases.
Researchers hoped that adding telomerase would keep cells dividing long enough to replace tissues lost to injury or disease. Normal cells often have proved impractical because they can only divide a limited number of times in culture, and once returned to the body they’re often too old to do much good. The limitation may be that normal cells do not produce active telomerase, which can rebuild the telomeres and keep cells from becoming senescent.
In fact, about a year ago, Jerry Shay and his colleagues at the University of Texas Southwestern Medical Center in Dallas showed that adding the enzyme to normal connective tissue cells called fibroblasts extends their life-span (Science NOW, 13 January 1998). These cells have now lived three times longer than normal in the lab, and they are still going strong. But because cancer cells contain telomerase and also live forever, scientists worried that the newly immortal cells would become malignant when implanted in humans.

Over the past decades, energy researchers have developed various promising solutions to limit the emission of greenhouse gases and source fuels or other chemicals more sustainably. These solutions include so-called carbon capture technologies and electrolyzers, devices that can capture carbon dioxide (CO2) and convert it into other valuable products, such as carbon monoxide (CO), methanol (CH₃OH), methane (CH₄) and various other compounds.
Some recently introduced solutions for converting CO2 into compounds that can be used as fuels or in industrial settings have achieved promising results. However, most of these devices only work if CO2 is purified (i.e., separated from other gases, contaminants and impurities). This additional purification step reduces the devices’ efficiency and can increase costs associated with their deployment, preventing their large-scale implementation.
An alternative method for the capture and conversion of CO2, known as reactive CO2 capture, could be more efficient and scalable than conventional approaches. This method combines the capture and conversion of CO2 in a single process, relying on compounds containing nitrogen (i.e., amine-based absorbents) to directly convert captured CO2 into desired compounds via electrochemical reactions.

Cancer is classified as the uncontrollable growth of mutated cells. There are different types of cancers that correlate to various tissues and organs. When individuals hear “cancer” they tend to think of breast cancer or another type of solid tumor. While about 90% of new cancer diagnoses are solid tumors, the remaining 10% are hematologic or blood cancers. Hematological malignancies affect the blood, bone marrow, and lymph nodes. Specific blood cancers include leukemia, lymphoma (Hodgkin’s and non-Hodgkin’s), and multiple myeloma. Around the world, these cancers account for about 7% of all cancer-related deaths, with a projection to increase to about 4.6 million cases in 2030.
Symptoms for hematological malignancies can vary and present in a wide range. Most common symptoms include fatigue, fever, weight loss, bruising, bleeding easily, anemia, low platelet count, and low white blood count. Many patients will also feel bone pain and muscle weakness accompanied by headaches and seizures. Current standard-of-care therapy include chemotherapy combined with another form of treatment. Dependent on the patient and the stage of the cancer, physicians can prescribe targeted therapies, immunotherapies, and stem cell transplants. However, some cancers find ways to resist therapy and continue to progress. Currently, many scientists are working to overcome barriers and improve therapy for patients with hematological malignancies.
A recent article in Science Advances, by Dr. Philippe Bousso and others, demonstrated that an immunotherapy can elicit a strong antitumor response by reprogramming malignant immune cells in lymphomas and leukemias. Bousso is an immunologist and head of the Dynamics of Immune Responses Unit at the Pasteur Institute in France. His work focuses on understanding immune responses in different diseases using innovative imaging approaches. Importantly, his work has helped the field of immunology redefine immune cell functions and showed how proteins secreted by immune cells can have an effect in distal locations throughout the body.


The plague of Akhetaten has long been cited as a possible explanation for the mysterious abandonment of ancient Egypt’s short-lived capital city. However, a comprehensive new archaeological analysis by researchers Dr. Gretchen Dabbs and Dr. Anna Stevens, published in the American Journal of Archaeology, analyzed the evidence for this plague and suggests it may never have affected Akhetaten at all.
Akhetaten, today known as Amarna, was built during the reign of Akhenaten, formerly known as Amonhotep IV. The pharaoh is known for his worship of a single deity, namely the sun god Aten.
In a possible attempt to distance himself from the old religion, he constructed a new royal residence and capital of the Egyptian kingdom called Akhetaten. However, the new capital was not occupied for long, lasting around 20 years before its near complete abandonment shortly after Akhenaten’s death.


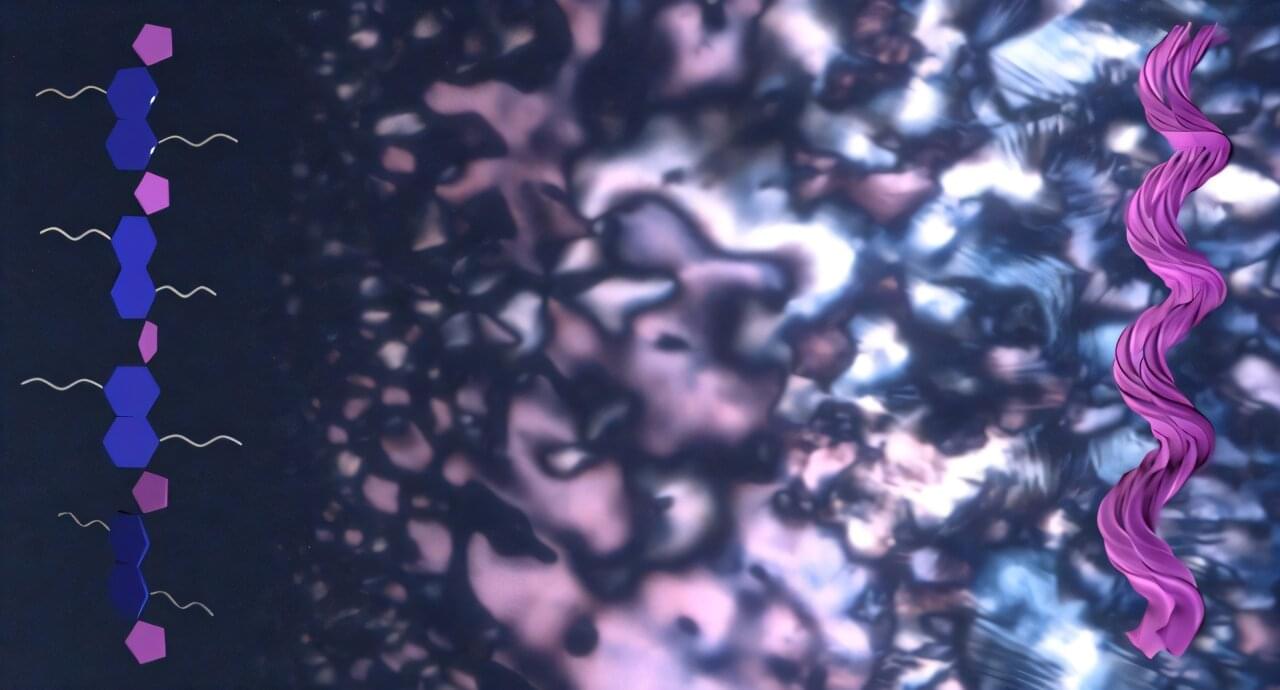
Chirality, a property where structures have a distinct left- or right-handedness, allows natural semiconductors to move charge and convert energy with high efficiency by controlling electron spin and the angular momentum of light. A new study has revealed that many conjugated polymers, long considered structurally neutral, can spontaneously twist into chiral shapes. This surprising behavior, overlooked for decades, could pave the way for development of a new class of energy-efficient electronics inspired by nature.
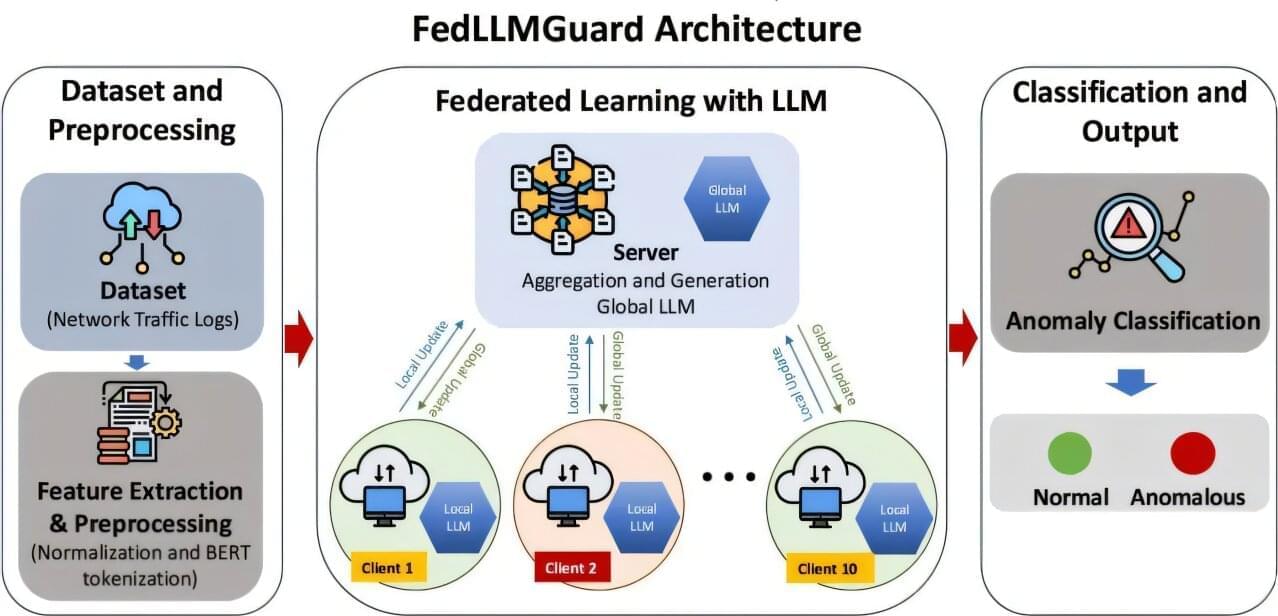
A framework for building tighter security into 5G wireless communications has been created by a Ph.D. student working with the University of Portsmouth’s Artificial Intelligence and Data Center.
With its greater network capacity and ability to rapidly transmit huge amounts of information from one device to another, 5G is a critical component of intelligent systems and services—including those for health care and financial services.
However, the dynamic nature of 5G networks, the high volumes of data shared and the ever changing types of information transmitted means that these networks are extremely vulnerable to cyber threats and increasing risks of attack.