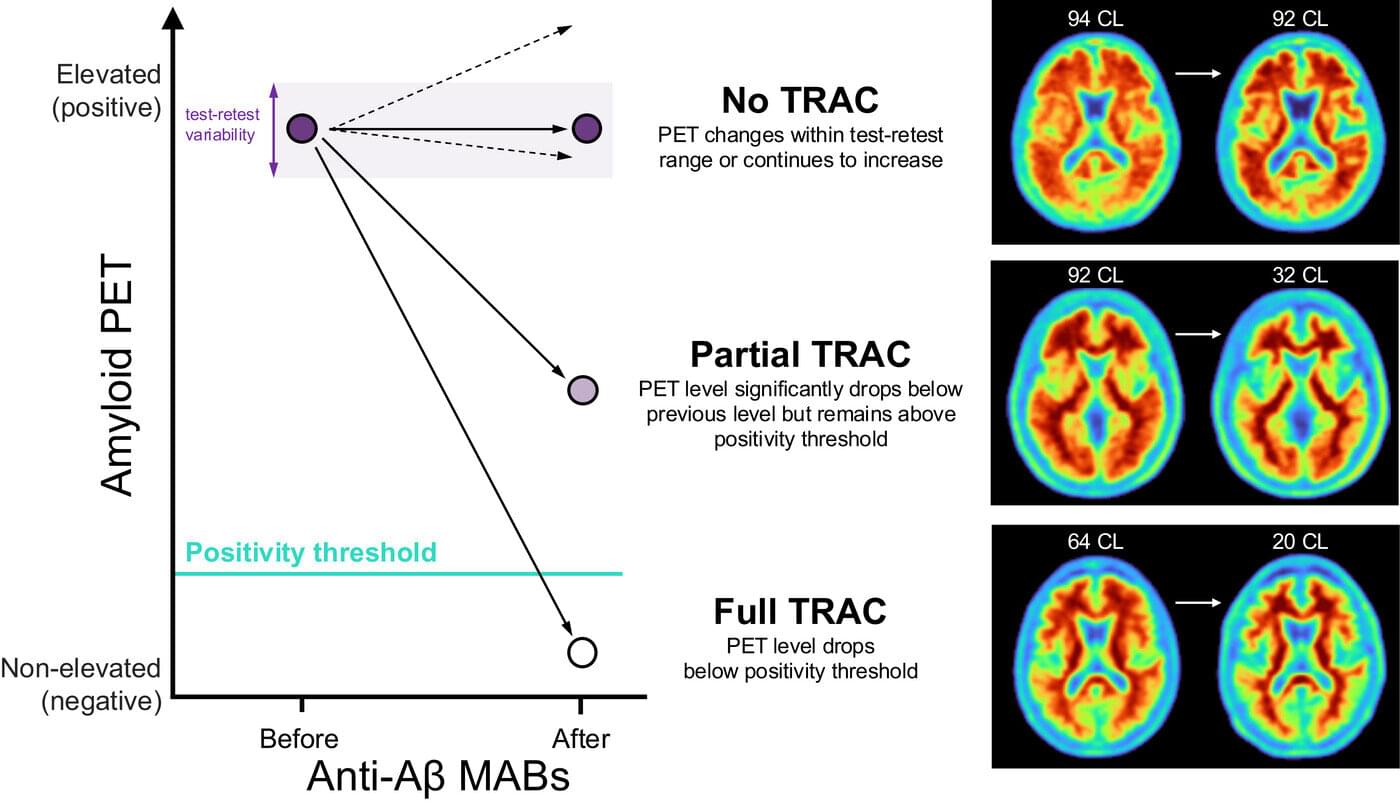
In the last few years, progress has been made in the fight against Alzheimer’s disease with a class of therapies called anti-amyloid antibodies (anti-Aβ). These monoclonal anti-Aβs are proteins made in a laboratory to stimulate the immune system to slow the progression of the disease by targeting amyloid plaques in the brain that are characteristic of Alzheimer’s.
Biomarkers, such as measures derived from PET scans that reflect amyloid plaques in the brain, were instrumental in FDA approval of anti-Aβ therapies, like lecanumab (Leqembi) and donanemab (Kisulna), and have been shown to reduce plaques in the brains of Alzheimer’s patients. Yet despite FDA approval, there is still a clinical need to better understand how to monitor the efficacy and safety of these treatments.
To this end, the Alzheimer’s Association convened a workgroup of scientists and clinicians with experience in Alzheimer’s disease, including clinical trials of anti-Aβ therapies and biomarkers, to propose a framework to characterize the response of patients receiving these treatments.